Abstract
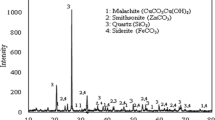
The extraction of copper from sulfate leaching liquor was investigated using Acorga M5640 commercial extractant in kerosene diluent. The effects of important factors such as the extractant concentration, contact time, liquor pH, and temperature were examined on the separation factor Cu/Fe and the extraction efficiency of copper. The results indicated that nearly 92.32% copper was extracted from the leach liquor at 10% (v/v) Acorga M5640 concentration, 1:1 aqueous/organic phase ratio, 20 min contact time, pH 2.5, and 25 °C temperature. Also, the further increase in the values determined had no positive impact on the extraction of copper. Under these conditions, the separation factor Cu/Fe was obtained about 29.64. The extraction mechanism was studied applying the plot of log(D) – 2 pH against log [extractant] and it proved the association of two moles of Acorga M5640 for the extraction of one mole of copper. McCabe–Thiele diagram was constructed and a single-step extraction process was found to obtain the maximum extraction. Additionally, thermodynamic data were investigated and the extraction process was endothermic and non-spontaneous at 298 K (ΔH = 27.05 and ΔG = 15.52 kJ mol−1). Moreover, the loaded organic phase was stripped and 80.94% copper was recovered after 20 min using 10% sulfuric acid concentration.












Similar content being viewed by others
References
Lemos LR, Santos IJB, Rodrigues GD et al (2012) Copper recovery from ore by liquid–liquid extraction using aqueous two-phase system. J Hazard Mater 237–238:209–214
Meterfi S, Meniai AH, Chikhi M (2012) Elimination of Cu (II) from aqueous solutions by liquid-liquid extraction. Test of sodium diethyldithiocarbamate (SDDT) as an extracting agent. Energy Procedia 18:1165–1174
Kasaie M, Bahmanyar H, Moosavian MA (2017) A kinetic study on solvent extraction of copper from sulfate solution with Cupromex-3302 using Lewis cell. J Environ Chem Eng 5:3044–3050
Deng J, Wen S, Yin Q, Wu D, Sun Q (2017) Leaching of malachite using 5-sulfosalicylic acid. J Taiwan Inst Chem Eng 71:20–27
Wieszczycka K, Wojciechowska I, Aksamitowski P (2019) Amphiphilic amidoxime ether as Cu(I) and Cu (II) extractant from waste etch solution. Sep Purif Technol 215:540–547
Nozhati RA, Azizi A (2020) Leaching of copper and zinc from the tailings sample obtained from a porcelain stone mine: feasibility, modeling, and optimization. Environ Sci Pollut Res 27:6239–6252
Agarwal S, Ferreira AE, Santos SM et al (2010) Separation and recovery of copper from zinc leach liquor by solvent extraction using Acorga M5640. Int J Miner Process 97:85–91
Li H, Eksteen J, Oraby E (2018) Hydrometallurgical recovery of metals from waste printed circuit boards (WPCBs): current status and perspectives–a review. Resour Conserv Recycl 139:122–139
Davenport WG, King M, Schlesinger M et al (2002) Chapter 18–solvent extraction transfer of cu from leach solution to electrolyte, extractive metallurgy of copper. Pergamon, Oxford, pp 307–325
Pradhan S, Mishra S (2015) A review on extraction and separation studies of copper with various commercial extractants. Metall Res Technol 112:202
El-Nadi YA (2017) Solvent extraction and its applications on ore processing and recovery of metals: classical approach. Sep Purif Rev 46:195–215
Tanda BC, Oraby EA, Eksteen JJ (2017) Recovery of copper from alkaline glycine leach solution using solvent extraction. Sep Purif Technol 187:389–396
Wang Y, Zhang Z, Kuang S, Wu G, Li Y, Li Y, Liao W (2018) Selective extraction and recovery of copper from chloride solution using Cextrant 230. Hydrometallurgy 181:16–20
Aksamitowski P, Filipowiak K, Wieszczycka K (2019) Selective extraction of copper from Cu-Zn sulfate media by new generation extractants. Sep Purif Technol 222:22–29
Ruiz MC, Risso J, Seguel J, Padilla R (2020) Solvent extraction of copper from sulfate-chloride solutions using mixed and modified hydroxyoxime extractants. Miner Eng 146:106109
El-Hefny NE, Daoud JA (2007) Extraction of copper (II) by CYANEX 302 in kerosene from different aqueous media. Solvent Extr Ion Exch 259:831–843
Reddy BR, Park KH, Mohapatra D (2007) Process development for the separation and recovery of copper from sulphate leach liquors of synthetic Cu–Ni–Co–Fe matte using LIX 84 and LIX 973N. Hydrometallurgy 87:51–57
Watling HR, Perrot FA, Shiers DW, Grosheva A, Richards TN (2009) Impact of the copper solvent extraction reagent LIX 984N on the growth and activity of selected acidophiles. Hydrometallurgy 95:302–307
Mishra S, Devi NB (2011) Extraction of copper (II) from hydrochloric acid solution by Cyanex 921. Hydrometallurgy 107:29–33
Devi NB, Mishra S (2012) Liquid-liquid extraction of copper (II) from chloride media by Cyanex 923 in kerosene. J South Afr Inst Min Metall 112:859–864
Lu J, Dreisinger D (2013) Solvent extraction of copper from chloride solution I: extraction isotherms. Hydrometallurgy 137:13–17
Jha MK, Gupta D, Choubey PK, Kumar V, Jeong J, Lee JC (2014) Solvent extraction of copper, zinc, cadmium and nickel from sulfate solution in mixer settler unit (MSU). Sep Purif Technol 122:119–127
Agarwal S, Reis MTA, Ismael MRC et al (2015) Extraction of Cu (II) with Acorga M5640 using hollow fibre liquid membrane. Chem Pap 69:679–689
Sombhatla SS, Kumar A, Mashruwala S, Rokkam KK, Shukla A (2016) Comparative study of organic solvents for extraction of copper from ammoniacal carbonate solution. Hydrometallurgy 166:94–97
Vasilyev F, Virolainen S, Sainio T (2017) Modeling the phase equilibrium in liquid–liquid extraction of copper over a wide range of copper and hydroxyoxime extractant concentrations. Chem Eng Sci 171:88–99
Feizollahi S, Azizi A (2018) Solvent extraction of copper from an industrial sulfate liquor using Chemorex CP-150. JME 9:905–916
Jiang F, Yin S, Zhang L, Peng J, Ju S, Miller JD, Wang X (2018) Solvent extraction of Cu (II) from sulfate solutions containing Zn (II) and Fe (III) using an interdigital micromixer. Hydrometallurgy 177:116–122
Elizalde MP, Rúa MS, Menoyo B, Ocio A (2019) Solvent extraction of copper from acidic chloride solutions with LIX 84. Hydrometallurgy 183:213–220
Soeezi A, Abdollahi H, Shafaei SZ et al (2020) Extraction and stripping of Cu and Ni from synthetic and industrial solutions of Sarcheshmeh copper mine containing Cu, Ni, Fe and Zn ions. Trans Nonferrous Metals Soc China 30:518–534
Sridhar V, Verma JK (2011) Extraction of copper, nickel and cobalt from the leach liquor of manganesebearing sea nodules using LIX 984N and ACORGA M5640. Miner Eng 24:959–962
Alguacil FJ, Lopez FA, Garcia-Diaz I (2012) Copper removal from acidic wastewaters using 2-hydroxy-5-nonylbenzaldehyde oxime as ionophore in pseudo-emulsion membrane with strip dispersion (PEMSD) technology. J Ind Eng Chem 18:255–259
Yang R, Wang S, Duan H, Yuan X, Huang Z, Guo H, Yang X (2016) Efficient separation of copper and nickel from ammonium chloride solutions through the antagonistic effect of TRPO on Acorga M5640. Hydrometallurgy 163:18–23
Wang L, Li Q, Sun X, Wang L (2019) Separation and recovery of copper from waste printed circuit boards leach solution using solvent extraction with Acorga M5640 as extractant. Sep Sci Technol 54:1302–1311
Agarwal S, Reis MTA, Ismael MRC, Correia MJN, Carvalho JMR (2012) Modelling of the extraction equilibrium of copper from sulfate solutions with Acorga M5640. Solvent Extr Ion Exch 30:536–551
Deep A, Kumar P, Carvalho JMR (2010) Recovery of copper from zinc leaching liquor using Acorga M5640. Sep Purif Technol 76:21–25
Li L, Wang Y, An W, Bao S (2017) Effect of the structure of alkyl salicylaldoxime on extraction of copper (II). Minerals 7:61
Tobiasz A, Walas S, Landowska L, Konefał-Góral J (2012) Improvement of copper FAAS determination conditions via preconcentration procedure with the use of salicylaldoxime complex trapped in polymer matrix. Talanta 96:82–88
Alguacil FJ, Alonso M, López-Delgado A et al (2004) Modelling copper (II) liquid-liquid extraction: the system Acorga M5640-Exxsol D100-CuSO4-H2SO4. J Chem Res Synop 3:196–197
Tait BK (1992) The extraction of some base metal ions by Cyanex 301, Cyanex 302, and their binary extractant mixtures with Aliquat 336. Solvent Extr Ion Exch 10:799–809
Fouad EA (2009) Separation of copper from aqueous sulfate solutions by mixtures of Cyanex 301 and LIX® 984N. J Hazard Mater 166:720–727
Aminian H, Bazin C (2000) Solvent extraction equilibria in copper (II)-iron (III)-LIX® 984 system. Miner Eng 13:667–672
Barik G, Nathsarma KC, Sarangi K (2013) Recovery of copper from a waste heat boiler dust leach liquor using LIX 84I and LIX 622N. Solvent Extr Ion Exch 31:198–209
Acknowledgments
The authors would like to appreciate the Bardeskan Nasim copper mine for providing the required samples.
Funding
The authors received financial support from the Shahrood University of Technology.
Author information
Authors and Affiliations
Contributions
The experiments were conducted via I. Nozari. The experimental design and interpretation of the results was carried out by A. Azizi.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nozari, I., Azizi, A. An Investigation into the Extraction Behavior of Copper from Sulfate Leach Liquor Using Acorga M5640 Extractant: Mechanism, Equilibrium, and Thermodynamics. Mining, Metallurgy & Exploration 37, 1673–1680 (2020). https://doi.org/10.1007/s42461-020-00280-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-020-00280-z