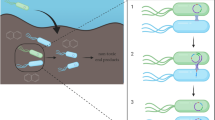
Abstract
It is well documented that bacteria can readily exchange genetic information under artificial conditions typically used in most laboratory studies as well as to some extent in nature. The three mechanisms by which such genetic exchange can occur are transformation, transduction and conjugation. Transformation is the uptake of free DNA into a cell from the surrounding environment, while bacterial viruses mediate the exchange of genetic material during transduction and conjugation involves the direct transfer of DNA during cell-to-cell contact. In most cases, plasmids mediate the transfer of DNA during conjugation events, although chromosomal transfer can also occur. This review will focus mainly on plasmids and the role of conjugation in marine sediment microbial communities. Plasmids, although often dispensible, provide a unique plasticity to an individual host cell or to an entire microbial community ‘genome’. Specifically, plasmid-encoded traits mobilized throughout microbial communities can provide a means of rapid adaptation to changing environmental conditions. Examples of such adaptation can be seen in the increased frequencies of catabolic plasmids and antibiotic and heavy metal resistance plasmids within microbial populations upon exposure to selective pressures. Presently, the view of plasmid diversity and horizontal transfer dynamics is predominantly based on broad- and narrow-host-range plasmids isolated from bacteria of clinical and animal origins. While the exchange of plasmids is most likely an important mechanism by which bacterial populations in clinical environments can evolve and adapt, there remains a general lack of information regarding the role of plasmid-mediated transfer in marine ecosystems and how indigenous plasmids impact the microbial community structure and function. The combined application of molecular biology and microbial ecology techniques is providing new approaches to address the ecological role of plasmids in marine environments.
Similar content being viewed by others
References
Angles, M. L., K. C. Marshall & A. E. Goodman, 1993. Plasmid transfer between marine bacteria in the aqueous phase and biofilms in reactor microcosms. Appl. envir. Microbiol. 59: 843–850.
Aviles, M., J. C. Codina, A. Perez-Garcia, F. Cazorla, P. Romero & A. de Vicente, 1993. Occurrence of resistance to antibiotics and metals and of plasmids in bacterial strains isolated from marine environments. Wat. Sci. Tech. 27: 475–478.
Barkay, T., N. Kroer, L. D. Rasmussen & S. J. Sorensen, 1995. Conjugal transfer at natural population densities in a microcosm simulating an estuarine environment. FEMS Microbiol. Ecol. 16: 43–54.
Barns, S. M., R. E. Fundyga, M.W. Jeffries & N. R. Pace, 1994. Remarkable archaeal diversity detected in a Yellowstone National Park hot spring environment. Proc. natl. Acad. Sci. U.S.A. 91: 1609–1613.
Baya, A. M., P. R. Brayton, V. L. Brown, D. J. Grimes, E. Russek-Cohen & R. R. Colwell, 1986. Coincident plasmids and antimicrobial resistance in marine bacteria isolated from polluted and unpolluted Atlantic Ocean samples. Appl. envir. Microbiol. 51: 1285–1292.
Belliveau, B. H., M. E. Starodub & J. T. Trevors, 1991. Occurrence of antibiotic and metal resistance and plasmids in Bacillus strains isolated from marine sediment. Can. J. Microbiol. 37: 513–520.
Benson, S. & J. Shapiro, 1978. TOL is a broad-host-range plasmid. J. Bact. 135: 278–280.
Birnboim, H. C. & J. Doly, 1979. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 7: 1513–1523.
Bukhari, A. I., J. A. Shapiro & S. L. Adhya, 1977. DNA insertion elements, plasmids, and episomes. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y., U.S.A.
Chamier, B., M. G. Lorenz & W. Wackernagel, 1993. Natural transformation of Acinetobacter calcoaceticus by plasmid DNA adsorbed on sand and groundwater aquifer material. Appl. envir. Microbiol. 59: 1662–1667.
Cook, M.A. and P.A. Sobecky, unpublished data.
Couturier, M. F., F. Bex, P.L. Bergquist & W. K. Maas, 1988. Identi-fication and classification of bacterial plasmids. Microbiol. Rev. 52: 375–395.
Dahlberg, C., M. Bergström & M. Hermansson, 1998. In situ detection of high levels of horizontal plasmid transfer in marine bacterial communities. Appl. envir. Microbiol. 64: 2670–2675.
Dahlberg, C., C. Linberg, V. L. Torsvik & M. Hermansson, 1997. Conjugative plasmids isolated from bacteria in marine environments show various degrees of homology to each other and are not closely related to well-characterized plasmids. Appl. envir. Microbiol. 63: 4692–4697.
Datta, N., 1979. Plasmid classification: incompatibility grouping. In K. N. Timmis & A. Puhler (ed.), Plasmids of Medical, Environmental and Commercial Importance. Elsevier/North-Holland Biomedical Press, Amsterdam, The Netherlands. 3–11.
Datta, N. & R. W. Hedges, 1972. Host ranges of R factors. J. Gen. Microbiol. 70: 453–460.
Davey, R. B., P. I. Bird, S. M. Nikoletti, J. Prazkier & J. Pittard, 1984. The use of mini-gal plasmids for rapid incompatability grouping of conjugative R plasmids. Plasmid 11: 234–242.
DeLong, E. F., 1992. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. U.S.A. 89: 5685–5689.
Delong, E. F., 1997. Marine microbial diversity: the tip of the iceberg. Trends Biotechnol. 15: 203–207.
Erauso, G., S. Marsin, N. Benbouzid-Rollet, M.-F. Baucher, T. Barbeyron, Y. Zivanovic, D. Prieur & P. Forterre, 1996. Sequence of plasmid pGT5 from the Archaeon Pyrococcus abyssi: evidence for rolling-circle replication in a hyperthermophile. J. Bact. 178: 3232–3237.
Frischer, M. E., G. J. Stewart & J. H. Paul, 1994. Plasmid transfer to indigenous marine bacterial populations by natural transformation. FEMS Microbiol. Ecol. 15: 127–136.
Fry, J. C., 1994. Genetic transfer in water. Presented at the Juan March Centre for International Meetings on Biology, Sevilla, Spain, 14–16 February, 1994.
Gauthier, M. J., F. Cauvin & J.-P. Breittmayer, 1985. Influence of salts and temperature on the transfer of mercury resistance from a marine pseudomonad to Escherichia coli. Appl. envir. Microbiol. 50: 38–40.
Giovannoni, S. J., T. B. Britschgi, C. L. Moyer & K. G. Field, 1990. Genetic diversity in Sargasso Sea bacterioplankton. Nature 345: 60–63.
Glassman, D. L. & L. A. McNicol, 1981. Plasmid frequency in natural populations of estuarine microorganisms. Plasmid 5: 231.
Goodman, A. E., E. Hild, K. C. Marshall & M. Hermansson, 1993. Conjugative plasmid transfer between bacteria under simulated marine oligotrophic conditions. Appl. envir. Microbiol. 59: 1035–1040.
Goodman, A. E., K. C. Marshall & M. Hermansson, 1994. Gene transfer among bacteria under conditions of nutrient depletion in simulated and natural aquatic environments. FEMS Microbiol. Ecol. 15: 55–60.
Guiney, D. G., 1993. Broad host range conjugative and mobilizable plasmids in gram-negative bacteria. In Don Clewell (ed.), Bacterial Conjugation. Plenum Press, N.Y., U.S.A. 75–103.
Hada, H. S. & R. K. Sizemore, 1981. Incidence of plasmids in marine Vibrio spp. isolated from an oil field in the northwestern Gulf of Mexico. Appl. envir. Microbiol. 41: 199–202.
Hansen, C. L., G. Zwolinsk, D. Martin & J. W. Williams, 1984. Bacterial removal of mercury from sewage. Biotechnol. Bioeng. 26: 1330–1303.
Helinski, D. R., A. E. Toukdarian & R. P. Novick, 1996. Replicaton control and other stable maintenance mechanisms of plasmids. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechtger & H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. American Society for Microbiology, Washington, D.C., U.S.A.: 2295–2324.
Hermansson, M., G. W. Jones & S. Kjelleberg, 1987. Frequency of antibiotic and heavy metal resistance, pigmentation, and plasmids in bacteria of the marine air-water interface. Appl. envir. Microbiol. 53: 2338–2342.
Hermansson, M. & C. Linberg, 1994. Gene transfer in the marine environment (minireview). FEMS Microbiol Ecol. 15: 47–54.
Hill, K. E., A. J. Weightman & J. C. Fry, 1992. Isolation and screening of plasmids from the epilithon which mobilize recombinant plasmid pD10. Appl. envir. Microbiol. 58: 1292–1300.
Kinkle, B. K., Sadowsky, M. J., E. L. Schmidt & W. C. Koskinen, 1993. Plasmids pJP4 and R68.45 can be transferred between populations of bradyrhizobia in soil. Appl. envir. Microbiol. 59: 1762–1766.
Kobayashi, N. & M. J. Bailey, 1994. Plasmids isolated from the sugar beet phyllosphere show little or no homology to molecular probes currently available for plasmid typing. Microbiology 140: 289–296.
Kobori, H., C. W. Sullivan & H. Shizuya, 1984. Bacterial plasmids in Antarctic natural assemblages. Appl. envir. Microbiol. 48: 515–518.
Leahy, J. G., C. C. Somerville, K. A. Cunningham, G. A. Adamantiades, J. J. Byrd & R. R. Colwell, 1990. Hydrocarbon mineralization in sediments and plasmid incidence in sediment bacteria from the Campeche Bank. Appl. envir. Microbiol. 56: 1565–1570.
Lilley, A. K., M. J. Bailey, M. J. Day & J. C. Fry, 1996. Diversity of mercury resistance plasmids obtained by exogenous isolation from the bacteria of sugar beet in three successive years. FEMS Microbiol. Ecol. 2: 211–227.
Maruyama, A., M. Oda & T. Higashihara, 1993. Abundance of virus-sized non-DNase-digestible DNA (coated DNA) in eutrophic seawater. Appl. envir. Microbiol. 3: 712–718.
Novick, R. P., R. C. Clowes, S. N. Cohen, R. Curtiss III, N. Datta & S. Falkow, 1976. Uniform nomenclature for bacterial plasmids: a proposal. Bact. Rev. 40: 168–189.
Novick, R. P., 1987. Plasmid incompatibility. Microbiol. Rev. 51: 381–395.
Ogunseitan, O. A., G. S. Sayler & R. V. Miller, 1990. Dynamic interactions between Pseudomonas aeruginosa and bacteriophages in lakewater. Microb. Ecol. 19: 171–185.
O'Morchoe, S., O. Ogunseitan, G. S. Sayler & R. V. Miller, 1988. Conjugal transfer of R68.45 and FP5 between Pseudomonas aeruginosa strains in a freshwater environment. Appl. envir. Microbiol. 54: 1923–1929.
Otto, K., D. Weichart & S. Kjelleberg, 1997. Plasmid transfer between marine Vibrio strains during predation by the het18 erotrophic microflagellate Cafeteria roenbergensis. Appl. envir. Microbiol. 63: 749–752.
Paul, J. H., M. E. Frisher & J. M. Thurmond, 1991. Gene transfer in marine water column and sediment microcosms by natural plasmid transformation. Appl. envir. Microbiol. 57: 1509–1515.
Pickup, R. W., 1989. Related plasmids found in an English Lake District stream. Microb. Ecol. 18: 211–220.
Riley, M. A. & D. M. Gordon, 1992. A survey of Col plasmids in natural isolates of Escherichia coli and an investigation into the stability of Col-plasmid lineages. J. Gen. Microbiol. 138: 1345–1352.
Rochelle, P. A., J. C. Fry & M. J. Day, 1989. Factors affecting conjugal transfer of plasmids encoding mercury resistance from pure cultures and mixed bacterial suspensions of epilithic bacteria. J. gen. Microbiol. 135: 409–424.
Sandaa, R.-A., V. L. Torsvik & J. Goksoyr, 1992. Transferable drug resistance in bacteria from fish-farm sediments. Can. J. Microbiol. 38: 1061–1065.
Saye, D. J., O. Ogunseitan, G. S. Sayler & R. V. Miller, 1990. Transduction of linked chromosomal genes between Pseudomonas aeruginosa strains during incubation in situ in a freshwater habitat. Appl. envir. Microbiol. 56: 140–145.
Sayler, G. S., S. W. Hooper, A. C. Layton & J. H. King, 1990. Catabolic plasmids of environmental and ecological significance. Microb. Ecol. 19: 1–20.
Schutt, C., 1989. Plasmids in the bacterial assemblage of a dystrophic lake: evidence for plasmid-encoded nickel resistance. Microb. Ecol. 17: 49–62.
Selander, R. K., D. A. Caugant & T. S. Whittam, 1987. Genetic structure and variation in natural populations of Escherichia coli. In Neidhardt, F. C., J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechtger & H. E. Umbarger (eds), Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. American Society for Microbiology, Washington, D.C., U.S.A.: 1625–1648.
Simon, R. D., M. Shilo & J. W. Hastings, 1982. The absence of a correlation between plasmids and luminescence in marine luminous bacteria. Curr. Microbiol. 7: 175–180.
Sizemore, R. K. & R. R. Colwell, 1977. Plasmids carried by antibiotic resistant marine bacteria. Antimicrob. Agents Chemother. 12: 372–382.
Sobecky, P. A., T. J. Mincer, M. C. Chang & D. R. Helinski, 1997. Plasmids isolated from marine sediment microbial communities contain replication and incompatibility regions unrelated to those of known plasmid groups. Appl. envir. Microbiol. 63: 888–895.
Sobecky, P. A., T. J. Mincer, M. C. Chang, A. E. Toukdarian & D. R. Helinski, Isolation of broad-host-range replicons from marine sediment bacteria. Appl. envir. Microbiol. 64: 2822–2830.
Sobecky, P. A., M. A. Schell, M. A. Moran & R. E. Hodson, 1996. Impact of a genetically engineered bacterium with enhanced alkaline phosphatase activity on marine phytoplankton communities. Appl. envir. Microbiol. 62: 6–12.
Stretton, S., S. Techkarnjanaruk, A. M. McLennan & A. E. Goodman, 1998. Use of green fluorescent protein to tag and investigate gene expression in marine bacteria. Appl. envir. Microbiol. 64: 2554–2559.
Thomas, C. M. & D. R. Helinski, 1989. Vegetative replication and stable inheritance of IncP plasmids. In C. M. Thomas (ed.), Promiscuous Plasmids of Gram-Negative Bacteria. Academic Press, London: 1–25.
Top, E., I. De Smet, W. Verstraete, R. Dijkmans & M. Mergeay, 1994. Exogenous isolation of mobilizing plasmids from polluted soils and sludges. Appl. envir. Microbiol. 60: 831–839.
Trevors, J. T., T. Barkay & A. W. Bourquin, 1987. Gene transfer among bacteria in soil and aquatic environments: a review. Can. J. Microbiol. 33: 191–198.
Van Elsas, J. D., B. B. McSpadden-Gardener, A. C. Wolters & E. Smit, 1998. Isolation, characterization, and transfer of cryptic gene-mobilizing plasmids in the wheat rhizosphere. Appl. envir. Microbiol. 64: 880–889.
Vieria, J. & J. Messing, 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19: 259–268.
Willetts, N. & B. Wilkins, 1984. Processing of plasmid DNA during bacterial conjugation. Microbiol. Rev. 48: 24–41.
Wortman, A. T. & R. R. Colwell, 1988. Frequency and characteristics of plasmids in bacteria isolated from deep sea Amphipods. Appl. envir. Microbiol. 54: 1284–1288.
Zawadzki, P., M. A. Riley & F. M. Cohen, 1996. Homology among nearly all plasmids infecting three Bacillus species. J. Bacteriol. 178: 191–198.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sobecky, P.A. Plasmid ecology of marine sediment microbial communities. Hydrobiologia 401, 9–18 (1999). https://doi.org/10.1023/A:1003726024628
Issue Date:
DOI: https://doi.org/10.1023/A:1003726024628