Abstract
Purpose. The sodium dependence and effects of nucleoside and nucleobase transport inhibitors were determined to ascertain the role of sodium dependent nucleoside or nucleobase transporters in nitrofurantoin active transport across mammary epithelia.
Methods. Five lactating female rats received steady-state intravenous infusions of nitrofurantoin with and without the broad-based inhibitor dipyridamole. In the CIT3 murine model of lactation, 14C-nitrofurantoin basolateral to apical permeability was examined in the presence of varying sodium concentrations, purine and pyrimidine nucleosides and nucleobases, and dipyridamole.
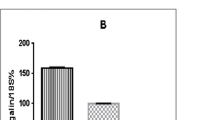
Results. Dipyridamole effectively inhibited 14C-nitrofurantoin flux across CIT3 cells, with Ki = 0.78 μM (95% C.I. = 0.11 to 5.3 μM) and significantly decreased the milk-to-serum ratio of nitrofurantoin from 29.2 ∓ 5.0 to 11.0 ∓ 6.3 without changing systemic clearance. Nitrofurantoin active transport was significantly inhibited by complete sodium replacement. Adenosine and guanosine significantly inhibited nitrofurantoin permeability (54.5 ∓ 2.6 (μl/hr)/cm2 and 50.7 ∓ 0.6 (μl/hr)/cm2, respectively, vs. control 90.5 ∓ 4.6 (μl/hr)/cm2) but uridine, thymidine, and the nucleobases had no effect.
Conclusions. Nitrofurantoin active transport was sodium dependent and inhibited by dipyridamole, adenosine, and guanosine, but known sodium dependent nucleoside or nucleobase transporters were not involved.
Similar content being viewed by others
REFERENCES
J. C. Fleishaker, N. Desai, and P. J. McNamara. Factors affecting the milk-to-plasma drug concentration ratio in lactating women: physical interactions with protein and fat. J. Pharm. Sci. 76:189–193 (1987).
J. C. Fleishaker and P. J. McNamara. In vivo evaluation in the lactating rabbit of a model for xenobiotic distribution into breast milk. J. Pharmacol. Exp. Ther. 244:919–924 (1988).
C. Y. Oo, R. J. Kuhn, N. Desai, and P. J. McNamara. Active transport of cimetidine into human milk. Clin. Pharmacol. Ther. 58:548–555 (1995).
F. W. Kari, R. Weaver, and M. C. Neville. Active transport of nitrofurantoin across the mammary epithelium in vivo. J. Pharmacol. Exp. Ther. 280: 664–668 (1997).
P. J. McNamara, J. A. Meece, and E. Paxton. Active transport of cimetidine and ranitidine into the milk of Sprague Dawley rats. J. Pharmacol. Exp. Ther. 277:1615–1621 (1996).
P. M. Gerk, R. J. Kuhn, N. Desai, and P. J. McNamara. Active transport of nitrofurantoin into human milk. Pharmacotherapy 21:669–675 (2001).
J. A. Belt, N. M. Marina, D. A. Phelps, and C. R. Crawford. Nucleoside transport in normal and neoplastic cells. Adv. Enzyme Regul. 33:235–252 (1993).
J. A. Thorn and S. M. Jarvis. Adenosine transporters. Gen. Pharmacol. 27:613–620 (1996).
J. Wang, M. E. Schaner, S. Thomassen, S.-F. Su, M. Piquette-Miller, and K. M. Giacomini. Functional and molecular characteristics of Na+-dependent nucleoside transporters. Pharm. Res. 14:1524–1532 (1997).
D. A. Griffith and S. M. Jarvis. Nucleoside and nucleobase transport systems of mammalian cells. Biochim. Biophys. Acta 1286:153–181 (1996).
C. E. Cass, J. D. Young, and S. A. Baldwin. Recent advances in the molecular biology of nucleoside transporters of mammalian cells. Biochem. Cell Biol. 76:761–770 (1998).
D. A. Griffith and S. M. Jarvis. High affinity sodium-dependent nucleobase transport in cultured renal epithelial cells (LLCPK1). J. Biol. Chem. 268:20085–20090 (1993).
D. A. Griffith and S. M. Jarvis. Characterization of a sodiumdependent concentrative nucleobase-transport system in quineapig kidney cortex brush-border membrane vesicles. Biochem. J. 303:901–905 (1994).
M. Shayeghi, R. Akerman, and S. M. Jarvis. Nucleobase transport in opossum kidney epithelial cells and Xenopus laevis oocytes: the characterization, structure-activity relationship and-independent hypoxanthine uptake. Biochim. Biophys. Acta 1416:109–118 (1999).
C. B. Washington and K. M. Giacomini. Mechanisms of nucleobase transport in rabbit choroid plexus. Evidence for a Na(+)-dependent nucleobase transporter with broad substrate selectivity. J. Biol. Chem. 270:22816–22819 (1995).
H. de Koning and G. Diallinas. Nucleobase transporters (review). Mol. Membr. Biol. 17:75–94 (2000).
P. S. Haddock. Evidence for sodium-dependent hypoxanthine uptake in isolated guinea pig ventricular myocytes: stimulation by extracellular Ni 2+. Cardiovasc. Res. 30:130–137 (1995).
C. Faaland, J. Race, G. Ricken, F. Warner, W. Williams, and E. Holtzman. Molecular characterization of two novel transporters from human and mouse kidney and from LLC-PK1 cells reveals a novel conserved family that is homologous to bacterial and Aspergillus nucleobase transporters. Biochim. Biophys. Acta 1442:353–360 (1998).
R. Bendayan. Interaction of dipyridamole, a nucleotide transport inhibitor, with the renal transport of organic cations by LLCPK1 cells. Can. J. Physiol. Pharmacol. 75:52–56 (1997).
S. Ayesh, Y. M. Shao, and W. D. Stein. Co-operative, competitive and non-competitive interactions between modulators of Pglycoprotein. Biochim. Biophys. Acta 1316:8–18 (1996).
J. R. Bronk and J. G. Hastewell. The transport of pyrimidines into tissue rings cut from rat small intestine. J. Physiol. 382:475–488 (1987).
G. Pons, E. Rey, M.-O. Richard, F. Vauzelle, C. Francoual, C. Moran, P. d'Athis, J. Badoual, and G. Olive. Nitrofurantoin excretion in human milk. Dev. Pharmacol. Ther. 14:148–152 (1990).
V. S. Toddywalla, F. W. Kari, and M. C. Neville. Active transport of nitrofurantoin across a mouse mammary epithelial monolayer. J. Pharmacol. Exp. Ther. 280:669–676 (1997).
D. R. Newell, P. M. O'Connor, A. H. Calvert, and K. R. Harrap. The effect of the nucleoside transport inhibitor dipyridamole on the incorporation of [3H]thymidine in the rat. Biochem. Pharmacol. 35:3871–3877 (1986).
S. D. Patil and J. D. Unadkat. Sodium-dependent nucleoside transport in the human intestinal brush-border membrane. Am. J. Physiol. 272:G1314–1320 (1997).
S. A. Flanagan and K. A. Mecklin-Gill. Characterization of a novel Na+-dependent, guanosine-specific, nitrobenzylthioinosine-sensitive transporter in acute promyelocytic leukemia cells. J. Biol. Chem. 272:18026–18032 (1997).
M. Sundaram, S. Y. Yao, A. M. Ng, M. Griffiths, C. E. Cass, S. A. Baldwin, and J. D. Young. Chimeric constructs between human and rat equilibrative nucleoside transporters (hENT1 and rENT1) reveal hENT1 structural domains interacting with coronary vasoactive drugs. J. Biol. Chem. 273:21519–21525 (1998).
E. Hosoi, M. Hirose, S. Hamano, M. Morimoto, and Y. Kuroda. Effect of MDR antagonists on the cidal activity of vincristine for cells expressing MDR-1 is superior to those expressing MRP. Int. J. Oncol. 13:343–348 (1998).
T. Heming, C. Vanoye, and A. Bidani. Dipyridamole inhibition of HCO3-Cl- exchange in human erythrocytes. J. Pharmacol. Exp. Ther. 255:631–635 (1990).
P. G. Plagemann and C. Woffendin. Comparison of the equilibrium exchange of nucleosides and 3-O-methylglucose in human erythrocytes and of the effects of cytochalasin B, phloretin and dipyridamole on their transport. Biochim. Biophys. Acta 899:295–301 (1987).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerk, P.M., Hanson, L., Neville, M.C. et al. Sodium Dependence of Nitrofurantoin Active Transport Across Mammary Epithelia and Effects of Dipyridamole, Nucleosides, and Nucleobases. Pharm Res 19, 299–305 (2002). https://doi.org/10.1023/A:1014495018640
Issue Date:
DOI: https://doi.org/10.1023/A:1014495018640