Abstract
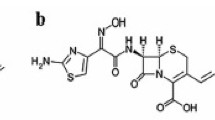
Cefprozil, a new oral cephalosporin, consists of a 90:10 cis:trans isomer mixture. Sensitive, specific and reproducible high performance liquid chromatographic methods have been developed for the simultaneous quantification of the two stereoisomers of cefprozil in plasma and urine samples from human and rats. Cephalexin acted as the internal standard. Plasma protein was precipitated with acetonitrile and trichloracetic acid with subsequent extraction of acetonitrile. After vortexing and centrifuging, the aqueous phase was injected onto a reverse phase C8 column. Urine samples were acidified with sodium acetate buffer (pH 3.8) and then directly injected onto a reverse phase C18 column. The detector was set at 280 nm. These methods were applied to determine protein binding of both isomers in human and rat sera, and to perform a pharmacokinetic study in human. Results showed that both isomers bound moderately to serum proteins with no interference by the other isomer. The pharmacokinetic study in human indicated that cefprozil was well absorbed and the cis and trans isomers have similar pharmacokinetics.
Similar content being viewed by others
REFERENCES
G. M. Eliopoulos, E. Reiszner, C. Wennersten, and R. C. Moellering. In vitro activity of BMY-28100, a new oral cephalosporin. Antimicrob. Agents Chemother. 31:653–656 (1987).
N. Chin and H. C. Neu. Comparative antibacterial activity of a new oral cephalosporin, BMY-28100. Antimicrob. Agents Chemother. 31:480–483 (1987).
F. Leitner, T. A. Pursiano, R. E. Buch, Y. H. Tsai, D. R. Chisholm, M. Misiek, J. V. Desiderio, and R. E. Kessler. BMY-28100, a new oral cephalosporin. Antimicrob. Agents Chemother. 31:238–242 (1987).
R. K. Scribner, M. I. Marks, and B. D. Finkhouse. In Vitro activity of BMY-28100 against common isolates from pediatric infections. Antimicrob. Agents Chemother. 31:630–631 (1987).
R. E. Kessler, T. A. Pursiano, V. Z. Rossomano, P. A. Doing, R. E. Buck, R. J. Deorio, and F. Leitner. IX International Congress of Infectious and Parasitic Diseases, Munich, July 20–26, 1986.
R. H. Barbhaiya, C. R. Gleason, W. C. Shyu, R. B. Wilber, R. R. Martin, and K. A. Pittman. Phase I study of single-dose BMY-28100, a new oral cephalosporin. Antimicrob. Agents Chemother. 34:202–205 (1990).
P. Prescott. An approximate test for outliers in linear models. Technometrics 17:129–132 (1975).
M. N. Dudley, W. C. Shyu, C. H. Nightingale, and R. Quintiliani. Effect of saturable serum protein binding on the pharmacokinetics of unbound cefonicid in humans. Antimicrob. Agents Chemother. 30:565–569 (1986).
S. Riegelman and P. Collier. The application of statistical moment theory to the evaluation of in vivo dissolution time and absorption time. J. Pharmacokin. Biopharm. 8:509–534 (1980).
M. Gibaldi and D. Perrier. Pharmacokinetics, 2nd ed., Marcel-Dekker, New York, 1982, pp. 409–417.
F. P. Tally, N. V. Jacobus, and M. Barza. In vitro activity and serum protein-binding of cefaclor. J. Antimicrob. Chemother. 5:159–165 (1979).
R. H. Barbhaiya, U. A. Shukla, C. R. Gleason, W. C. Shyu, R. B. Wilber, R. R. Martin, and K. A. Pittman. Phase I study of multiple-dose cefprozil and comparison with cefaclor. Antimicrob. Agents Chemother. 34:1198–1203 (1990).
R. H. Barbhaiya, U. A. Shukla, C. R. Gleason, W. C. Shyu, and K. A. Pittman. Comparison of cefprozil and cefaclor pharmacokinetics and tissue penetration. Antimicrob. Agents Chemother. 34:1204–1209 (1990).
R. H. Barbhaiya, U. A. Shukla, C. R. Gleason, W. C. Shyu, and K. A. Pittman. Comparison of the effects of food on the pharmacokinetics of cefprozil and cefaclor. Antimicrob. Agents Chemother. 34:1210–1213 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shyu, W.C., Shukla, U.A., Shah, V.R. et al. Simultaneous High-Performance Liquid Chromatographic Analysis of Cefprozil Diastereomers in a Pharmacokinetic Study. Pharm Res 8, 992–996 (1991). https://doi.org/10.1023/A:1015896722170
Issue Date:
DOI: https://doi.org/10.1023/A:1015896722170