Abstract
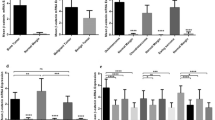
Several cytokines including members of the transforming growth factor-beta (TGF-β) and tumor necrosis factor (TNF) families have been implicated in the homing mechanism of breast cancer metastasis. We hypothesize that primary breast tumor tissues differentially express modulators of bone cell function and that this expression pattern contributes to their aggressive and metastatic potential and to their capacity to establish and grow in bone. We, therefore, examined the gene expression pattern of the TGF-β family members (inhibin/activin βA subunit (activin βA), inhibin α subunit, and bone morphogenetic protein-2 (BMP-2)), the TNF family members (receptor activator of NF-κB ligand (RANKL) and osteoprotegerin (OPG)), and osteopontin (OPN) in normal, non-invasive, invasive, and metastatic human breast cancer specimens. The mRNA transcript levels of these genes were quantified by reverse transcription (RT) and fluorescent-based kinetic PCR in 18 normal breast tissues, five ductal carcinoma in situ (DCIS), 24 primary breast tumor tissue, and five distant metastases. The mRNA transcript level of each gene was normalized to the amount of β-actin present in the samples. We observed differential gene expression of the selected TGF-β family members as well as OPN in breast cancer progression. The average gene expression of the putative tumor suppressor, inhibin α, did not significantly change in any of the tumor tissues examined compared to normal breast tissue. The mRNA level of BMP-2, a protein with anti-proliferative effects in breast cancer cell lines and involved in bone formation, significantly decreased in non-invasive, invasive, and liver metastatic breast tumor tissue compared to normal breast tissue. The gene expression of activin βA, a protein involved in cell proliferation and osteoclast induction, increased in invasive and bone metastatic tumor tissue compared to normal breast tissue. The mRNA level of OPN, a bone matrix protein associated with enhanced malignancy, increased in non-invasive, invasive, and liver and bone metastatic breast tumor tissue compared to normal breast tissue. In contrast, the average gene expressions of the TNF family members, RANKL and OPG, proteins involved in the regulation of osteoclastogenesis, were only slightly if at all changed in the different stage breast tumor tissues. These results suggest that differential gene expression of bone-related proteins, especially OPN, activin βA, and BMP-2, by primary breast tumor tissues may play a significant role in the invasiveness and metastatic potential of breast cancer.
Similar content being viewed by others
References
Martin TJ, Moseley JM: Mechanisms in the skeletal complications of breast cancer. Endocr Relat Cancer 7: 271–284, 2000
Coleman RE: Skeletal complications of malignancy. Cancer 80: 1588–1594, 1997
Yoneda T: Cellular and molecular mechanisms of breast and prostate cancer metastasis to bone. Eur J Cancer 34: 240–245, 1998
Guise TA: Molecular mechanisms of osteolytic bone metastases. Cancer 88: 2892–2898, 2000
Guise TA, Mundy GR: Cancer and bone. Endocr Rev 19: 18–54, 1998
Gillespie MT, Thomas RJ, Pu ZY, Zhou H, Martin TJ, Findlay DM: Calcitonin receptors, bone sialoprotein and osteopontin are expressed in primary breast cancers. Int J Cancer 73: 812–815, 1997
Mundy GR, Martin TJ: Pathophysiology of skeletal complic-ations of cancer. In: Born GVR, Cuatrecasas P, Herken H, Melmon K (eds) Handbook of Experimental Pharmacology. Vol 107, Springer-Verlag, Berlin, 1993, pp 642–671
Renier MA, Vereecken A, Buytaert P: Inhibins, activins and follistatins: a review of complex regulators of the reproductive system. Eur J Contracept Reprod Health Care 3: 129–135, 1998
Meunier H, Rivier C, Evans RM, Vale W: Gonadal and extragonadal expression of inhibin alpha, beta A, and beta B subunits in various tissues predicts diverse functions. Proc Natl Acad Sci USA 85: 247–251, 1988
Mather JP, Moore A, Li RH: Activins, inhibins, and follistat-ins: further thoughts on a growing family of regulators. Proc Soc Exp Biol Med 215: 209–222, 1997
Wada M, Shintani Y, Kosaka M, Sano T, Hizawa K, Saito S: Immunohistochemical localization of activin A and follistatin in human tissues. Endocr J 43: 375–385, 1996
de Jong FH, Grootenhuis AJ, Klaij IA, Van Beurden WM: Inhibin and related proteins: localization, regulation, and effects. Adv Exp Med Biol 274: 271–293, 1990
Srivastava P, Russo J, Russo IH: Inhibition of rat mammary tumorigenesis by human chorionic gonadotropin associated with increased expression of inhibin. Mol Carcinog 26: 10–19, 1999
Lojun S, Bao S, Lei ZM, Rao CV: Presence of functional luteinizing hormone/chorionic gonadotropin (hCG) receptors in human breast cell lines: implications supporting the premise that hCG protects women against breast cancer. Biol Reprod 57: 1202–1210, 1997
Russo IH, Russo J: Role of hCG and inhibin in breast cancer (review). Int J Cancer 4: 297–306, 1994
Matzuk MM, Finegold MJ, Su JG, Hsueh AJ, Bradley A: Alpha-inhibin is a tumour-suppressor gene with gonadal specificity in mice. Nature 360: 313–319, 1992
Yu J, Dolter KE: Production of activin A and its roles in inflammation and hematopoiesis. Cytokines Cell Mol Ther 3: 169–177, 1997
Fuller K, Bayley KE, Chambers TJ: Activin A is an essential cofactor for osteoclast induction. Biochem Biophys Res Commun 268: 2–7, 2000
Robinson GW, Hennighausen L: Inhibins and activins regulate mammary epithelial cell differentiation through mesenchymal-epithelial interactions. Development 124: 2701–2708, 1997
Kalkhoven E, Roelen BA, de Winter JP, Mummery CL, van den Eijnden-van Raaij AJ, van der Saag PT, van der Burg B: Resistance to transforming growth factor beta and activin due to reduced receptor expression in human breast tumor cell lines. Cell Growth Differ 6: 1151–1161, 1995
Ying SY, Zhang Z: Expression and localization of inhibin/ activin subunits and activin receptors in MCF-7 cells, a human breast cancer cell line. Breast Cancer Res Treat 37: 151–160, 1996
Liu QY, Niranjan B, Gomes P, Gomm JJ, Davies D, Coombes RC, Buluwela L: Inhibitory effects of activin on the growth and morpholgenesis of primary and trans-formed mammary epithelial cells. Cancer Res 56: 1155–1163, 1996
Hogan BL: Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev 10: 1580–1594, 1996
Clement JH, Marr N, Meissner A, Schwalbe M, Sebald W, Kliche KO, Hoffken K, Wölfl S: Bone morphogenetic protein 2 (BMP-2) induces sequential changes of Id gene expression in the breast cancer cell line MCF-7. J Cancer Res Clin Oncol 126: 271–279, 2000
Ghosh-Choudhury N, Woodruff K, Qi W, Celeste A, Abboud SL, Ghosh Choudhury G: Bone morphogenetic protein-2 blocks MDA MB 231 human breast cancer cell proliferation by inhibiting cyclin-dependent kinase-mediated retinoblastoma protein phosphorylation. Biochem Biophys Res Commun 272: 705–711, 2000
Ghosh-Choudhury N, Ghosh-Choudhury G, Celeste A, Ghosh PM, Moyer M, Abboud SL, Kreisberg J: Bone morphogen-etic protein-2 induces cyclin kinase inhibitor p21 and hypo-phosphorylation of retinoblastoma protein in estradiol-treated MCF-7 human breast cancer cells. Biochim Biophys Acta 1497: 186–196, 2000.
Arnold SF, Tims E, McGrath BE: Identification of bone morphogenetic proteins and their receptors in human breast cancer cell lines: importance of BMP2. Cytokine 11: 1031–1037, 1999
Soda H, Raymond E, Sharma S, Lawrence R, Cerna C, Gomez L, Timony GA, Von Hoff DD, Izbicka E: Antiproliferative effects of recombinant human bone morphogenetic protein-2 on human tumor colony-forming units. Anticancer Drugs 9: 327–331, 1998
Denhardt DT, Guo X: Osteopontin: a protein with diverse functions. Faseb J 7: 1475–1482, 1993
Brown LF, Papadopoulos-Sergiou A, Berse B, Manseau EJ, Tognazzi K, Perruzzi CA, Dvorak HF, Senger DR: Osteopontin expression and distribution in human carcinomas. Am J Pathol 145: 610–623, 1994
Bellahcène A, Castronovo V: Increased expression of osteonectin and osteopontin, two bone matrix proteins, in human breast cancer. Am J Pathol 146: 95–100, 1995
Bellahcène A, Castronovo V: Expression of bone matrix proteins in human breast cancer: potential roles in microcalcification formation and in the genesis of bone metastases. Bull Cancer 84: 17–24, 1997
Hirota S, Ito A, Nagoshi J, Takeda M, Kurata A, Takatsuka Y, Kohri K, Nomura S, Kitamura Y: Expression of bone matrix protein messenger ribonucleic acids in human breast cancers. Possible involvement of osteopontin in development of calcifying foci. Lab Invest 72: 64–69, 1995
Kim YW, Park YK, Lee J, Ko SW, Yang MH: Expression of osteopontin and osteonectin in breast cancer. J Korean Med Sci 13: 652–657, 1998
Horowitz MC, Xi Y, Wilson K, Kacena MA: Control of osteoclastogenesis and bone resorption by members of the TNF family of receptors and ligands. Cytokine Growth Factor Rev 12: 9–18, 2001
Senger DR, Perruzzi CA, Papadopoulos A: Elevated expression of secreted phosphoprotein I (osteopontin, 2ar) as a consequence of neoplastic transformation. Anticancer Res 9: 1291–1299, 1989
Singhal H, Bautista DS, Tonkin KS, O'Malley FP, Tuck AB, Chambers AF, Harris JF: Elevated plasma osteopontin in meta-static breast cancer associated with increased tumor burden and decreased survival. Clin Cancer Res 3: 605–611, 1997
Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ: Modulation of osteoclast differentiation and func-tion by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20: 345–357, 1999
Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL: The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 15: 2–12, 2000
Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ, Gillespie MT: Breast cancer cells interact with osteo-blasts to support osteoclast formation. Endocrinology 140: 4451–4458, 1999
Holland PM, Abramson RD, Watson R, Gelfand DH: Detection of specific polymerase chain reaction product by utilizing the 5 -3 exonuclease activity of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci USA 88: 7276–7280, 1991
Reimer T, Koczan D, Muller H, Friese K, Krause A, Thiesen HJ, Gerber B: Human chorionic gonadotrophin-beta transcripts correlate with progesterone receptor values in breast carcinomas. J Mol Endocrinol 24: 33–41, 2000
Bièche I, Laurendeau I, Tozlu S, Olivi M, Vidaud D, Lidereau R, Vidaud M: Quantitation of MYC gene ex-pression in sporadic breast tumors with a real-time reverse transcription-PCR assay. Cancer Res 59: 2759–2765, 1999
Bièche I, Onody P, Laurendeau I, Olivi M, Vidaud D, Lidereau R, Vidaud M: Real-time reverse transcription-PCR assay for future management of ERBB2-based clinical applications. Clin Chem 45: 1148–1156, 1999
Murata M, Eto Y, Shibai H, Sakai M, Muramatsu M: Erythroid differentiation factor is encoded by the same mRNA as that of the inhibin beta A chain. Proc Natl Acad Sci USA 85: 2434–2438, 1988
Mason AJ, Niall HD, Seeburg PH: Structure of two human ovarian inhibins. Biochem Biophys Res Commun 135: 957–964, 1986
Tanimoto K, Handa S, Ueno N, Murakami K, Fukamizu A: Structure and sequence analysis of the human activin beta A subunit gene. DNA Seq 2: 103–110, 1991
Mayo KE, Cerelli GM, Spiess J, Rivier J, Rosenfeld MG, Evans RM, Vale W: Inhibin A-subunit cDNAs from porcine ovary and human placenta. Proc Natl Acad Sci USA 83: 5849–5853, 1986
Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA: Novel regulators of bone formation: molecular clones and activities. Science 242: 1528–1534, 1988
Tabas JA, Zasloff M, Wasmuth JJ, Emanuel BS, Altherr MR, McPherson JD, Wozney JM, Kaplan FS: Bone morphogenetic protein: chromosomal localization of human genes for BMP1, BMP2A, and BMP3. Genomics 9: 283–289, 1991
Rao VV, Loffler C, Wozney JM, Hansmann I: The gene for bone morphogenetic protein 2A (BMP2A) is localized to human chromosome 20p12 by radioactive and nonradioactive in situ hybridization. Hum Genet 90: 299–302, 1992
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ: Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93: 165–176, 1998
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Boyle WJ et al.: Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89: 309–319, 1997
Hijiya N, Setoguchi M, Matsuura K, Higuchi Y, Akizuki S, Yamamoto S: Cloning and characterization of the human osteopontin gene and its promoter. Biochem J 303: 255–262, 1994
Dirnhofer S, Berger C, Untergasser G, Geley S, Berger P: Human beta-actin retropseudogenes interfere with RT-PCR. Trends Genet 11: 380–381, 1995
Lacroix M, Marie PJ, Body JJ: Protein production by osteoblasts: modulation by breast cancer cell-derived factors. Breast Cancer Res Treat 61: 59–67, 2000
Harada K, Shintani Y, Sakamoto Y, Wakatsuki M, Shitsukawa K, Saito S: Serum immunoreactive activin A levels in normal subjects and patients with various diseases. J Clin Endocrinol Metab 81: 2125–2130, 1996
Di Loreto C, Reis FM, Cataldi P, Zuiani C, Luisi S, Beltrami CA, Petraglia F: Human mammary gland and breast carcinoma contain immunoreactive inhibin/activin subunits: evidence for a secretion into cystic fluid. Eur J Endocrinol 141: 190–194, 1999
Kanatani M, Sugimoto T, Kaji H, Kobayashi T, Nishiyama K, Fukase M, Kumegawa M, Chihara K: Stimulatory ef-fect of bone morphogenetic protein-2 on osteoclast-like cell formation and bone-resorbing activity. J Bone Miner Res 10: 1681–1690, 1995
Tuck AB, O'Malley FP, Singhal H, Harris JF, Tonkin KS, Kerkvliet N, Saad Z, Doig GS, Chambers AF: Osteopontin expression in a group of lymph node negative breast cancer patients. Int J Cancer 79: 502–508, 1998
Sharp JA, Sung V, Slavin J, Thompson EW, Henderson MA: Tumor cells are the source of osteopontin and bone sialoprotein expression in human breast cancer. Lab Invest 79: 869–877, 1999
Tuck AB, Arsenault DM, O'Malley FP, Hota C, Ling MC, Wilson SM, Chambers AF: Osteopontin induces increased invasiveness and plasminogen activator expression of human mammary epithelial cells. Oncogene 18: 4237–4246, 1999
Kasugai S, Zhang Q, Overall CM, Wrana JL, Butler WT, Sodek J: Differential regulation of the 55 and 44 kDa forms of secreted phosphoprotein 1 (SPP-1, osteopontin) in normal and transformed rat bone cells by osteotropic hormones, growth factors and a tumor promoter. Bone Miner 13: 235–250, 1991
van der Pluijm, Vloedgraven H, Papapoulos S, Löwik C, Grzesik W, Kerr J, Robey PG: Attachment characteristics and involvement of integrins in adhesion of breast cancer cell lines to extracellular bone matrix components. Lab Invest 77: 665–675, 1997
Morony S, Capparelli C, Sarosi I, Lacey DL, Dunstan CR, Kostenuik PJ: Osteoprotegerin inhibits osteolysis and de-creases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Res 61: 4432–4436, 2001
Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T: Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med 191: 275–286, 2000
Elte JW, Bijvoet OL, Cleton FJ, van Oosterom AT, Sleeboom HP: Osteolytic bone metastases in breast carcinoma pathogenesis, morbidity and bisphosphonate treatment. Eur J Cancer Clin Oncol 22: 493–500, 1986
Clohisy DR, Palkert D, Ramnaraine ML, Pekurovsky I, Oursler MJ: Human breast cancer induces osteoclast activa-tion and increases the number of osteoclasts at sites of tumor osteolysis. J Orthop Res 14: 396–402, 1996
Pederson L, Winding B, Foged NT, Spelsberg TC, Oursler MJ: Identification of breast cancer cell line-derived paracrine factors that stimulate osteoclast activity. Cancer Res 59: 5849–5855, 1999
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reinholz, M.M., Iturria, S.J., Ingle, J.N. et al. Differential Gene Expression of TGF-β Family Members and Osteopontin in Breast Tumor Tissue: Analysis by Real-Time Quantitative PCR. Breast Cancer Res Treat 74, 255–269 (2002). https://doi.org/10.1023/A:1016339120506
Issue Date:
DOI: https://doi.org/10.1023/A:1016339120506