Abstract
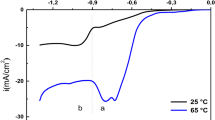
Linear potential sweep voltammetry was used to characterize the copper oxides grown on a metal substrate when exposed to a low pressure inductively coupled oxygen plasma. This study confirms the formation of a precursor oxide CuxO (x > 4), two copper(i) oxides Cu2-xO and Cu3O2 and copper(ii) oxide CuO. The electrochemical reduction curve of CuxO is characterized in aqueous solution (pH 9.2) by a minor peak near –0.5V vs SCE while the two Cu(i) oxides present one reduction peak at −0.8 VvsSCE and cannot be electrochemically separated; CuO is reduced to Cu(i) at −0.65V vs SCE. The reduction potentials of the copper(i) and copper(ii) oxides vary with the oxide layer thickness which increases with the time of exposure to the plasma and the injected electric power and decreases as the distance between the sample and the 1st coil increases for given treatment parameters. In addition, a mechanism is proposed for the reduction of thin films containing the copper(i) and copper(ii) oxides formed after plasma treatment.
Similar content being viewed by others
References
J. L. Brisset, S. Longchamp, P. Surbled and M. Vittecoq, Proc 4th International Symposium on High Pressure Low Temperature Plasma Chemistry, Bratislava, Slovakia (1993) p. 117.
A. Goldman and R. S. Sigmond, J. Electrochem. Soc. 132 (1985) 2842.
M. Carballeira, A. Carballeira and J. Y. Gal, Proceedings of the 14th International Conference on Electric Contacts, Paris, France (1988) p. 239.
J. Kúudela, V. Sobek, M. Luknárova P. Lukác and J. D. Skalný, Acta, Physica Univ. Comenianae 33 (1992) 209.
B. G. Bagley, L. H. Greene, J. M. Tarascon and G. W. Hull, Appl. Phys. Lett. 51 (1987) 622.
A. Yoshida, H. Tamura, S. Morohashi and S. Hasuo, ibid. 55 (1989) 2354.
R. L. Deutscher and R. Woods, J. Appl. Electrochem. 16 (1986) 413.
M. Lenglet, K. Kartouni and D. Delahaye, ibid. 21 (1991) 697.
J. M. Machefert, M. Lenglet, D. Blavette, A. Menand and A. D' Huysser, 'Structure and Reactivity of Surfaces', Elsevier Sciences Publishers B.V., Amsterdam (1989) p. 625.
B. Lefez, K. Kartouni, M. Lenglet, D. Rönnow and C. G. Ribbing, Surf. & Interface Anal. 22 (1994) 451.
E. Sutter, C. Fiaud and D. Lincot, Electrochim. Acta 38 (1993) 1471.
H. Pops and D. R. Hennessy, Wire J. 10 (1977) 50.
H. Strehblow and B. Titze, Electrochim. Acta, 25 (1980) 839.
M. R. Gennerro de Chialvo, S. L. Marchiano and A. J. Arvia, J. Appl. Electrochem. 14 (1984) 165.
U. R. Evans and A. Miley, Nature 139 (1937) 283.
[16] P. Pascal, Nouveau Traité de Chimie Minérale III, Masson, Paris (1957).
D. Personn and C. Leygraf, J. Electrochem. Soc. 140 (1993)1256.
J. Y. Malvault, J. Lopitaux, D. Delahaye and M. Lenglet, J. Appl. Electrochem. 25 (1995) 841.
H. Wieder and A. W. Czanderna, J. Phys. Chem. 66 (1962) 816.
E. G. Clarke and A. W. Czanderna, Surf. Sci. 49 (1975 529.
H. Neumeister and W. Jaenike, Z. Phys. Chem. B108 (1977) 217.
M. Lenglet and K. Kartouni, La Revue de Métallugie-CIT/ Science et Génie des Matériaux 12 (1993)1637.
S. Brahms, J. P. Dahl and S. Nikitine, J. Phys. C3-32 (1967) 28.
P. Marksteiner, P. Blaha and K. Schwarz, Z. Physik. B64 (1986) 119.
H. Wieder and A. W. Czanderna, J. Appl. Phys. 37 (1966) 184.
J. Bloem, Phil. Res. Rep. 13 (1958) 167.
C. K. Teh and F. L. Weichman, Can. J. Phys. 61 (1983) 1423.
R. G. Greenler, R. R. Rahn and J. P. Schwartz. J. Catal. 23 (1971) 42.
N. Bellakhal, Thèse, Université Paris VI (1995).
N. Bellakhal, K. Draou, B. Chéron, M. Lenglet and J. L. Brisset, Proceedings ISPC 12 Minneapolis, USA (1995) p.1583.
H. H. Strehblow and B. Titze, Electrochim. Acta, 25 (1980) 839.
S. M. Wilhelm, Y. Tanizawa, C.Y. Liu and N. Hackerman, Corros. Sci. 22 (1982) 791.
B. Millet, Thèse, Université Paris VI (1994).
E. Beucher, B. Lefez and M. Lenglet, Phys. Stat. Sol. 136 (1993) 139.
N. A. Tolstoi and V. A. Bonch-Bruevich, Sov. Phys. Solid State 13 (1971) 1135.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BELLAKHAL , N., DRAOU , K. & BRISSET , J.L. Electrochemical investigation of copper oxide films formed by oxygen plasma treatment. Journal of Applied Electrochemistry 27, 414–421 (1997). https://doi.org/10.1023/A:1018409620079
Issue Date:
DOI: https://doi.org/10.1023/A:1018409620079