Abstract
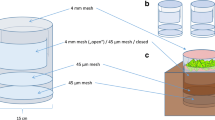
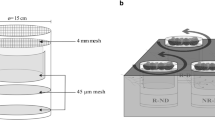
We studied the sensitivity of soil microbial communities and ecosystem processes to variation in the vertical and horizontal structure of decomposer food web under nitrogen poor and N-enriched conditions. Microcosms with humus and litter layer of boreal forest floor, birch seedlings infected with mycorrhizal fungi, and decomposer food webs with differing trophic group and species composition of soil fauna were constructed. During the second growing period for the birch, we irrigated half of the microcosms with urea solution, and the other half with de-ionised water to create two levels of N concentration in the substrate. During the experiment night time respirations of the microcosms were measured, and the water leached through the microcosms was analysed for concentration of mineral N, and nematode numbers. The microcosms were destructively sampled after 37 weeks for plant biomass and N uptake, structure of soil animal and microbial community (indicated by PLFA profiles), and physical and chemical properties of the humus and litter materials. Predatory mites and nematodes had a negative influence on the biomass of their microbivorous and microbi-detritivorous prey, and microbi-detritivores affected the biomass and community structure of microbes (indicated by PLFA-analysis). Moreover, predatory mites and nematodes increased microbial biomass and changed the microbial community structure. The decomposer food web structure affected also N uptake and growth of plants. Microbi-detritivorous fauna had a positive effect, whereas predators of microbial and detritus feeding fauna exerted a negative influence on plant N uptake and biomass production. The impact of a trophic group on the microbes and plant was also strongly dependent on species composition within the group. Nitrogen addition magnified the influence of food web structure on microbial biomass and plant N uptake. We suggest that addition of urea-N to the soil modified the animal-microbe interaction by increasing microbial growth and altering community structure of microbes. The presence of microbi-detritivores and predators reduced loss of carbon from the microcosms, and the food web structure influenced also water holding capacity of the materials. The changes in plant growth, nutrient cycling, size of N and C pools, and in the physical properties of the soil emphasize the importance and diversity of indirect consequences of decomposer food web structure.
Similar content being viewed by others
References
Abrahamsen G 1990 Influence of Cognettia sphagnetorum (Oligochaeta: Enchytraeidae) on nitrogen mineralization in homogenized mor humus. Biol. Fertil. Soils 9, 159–162.
Abrams P A, Menge B A, Mittelbach G G, Spiller D A and Yodzis P 1996 The role of indirect effects in food webs. In Food webs: integration of patterns and dynamics. Eds. G A Polis and K O Winemiller. pp 371–395. Chapman & Hall, New York.
Allen M F 1991 The ecology of mychorrhizae. Cambridge University Press, Cambridge.
Allen-Morley C R and Coleman D C 1989 Resilience of soil biota in various food webs to freezing perturbations. Ecology 70, 1127–1141.
Alphei J, Bonkowski M and Scheu S 1996 Protozoa, Nematoda and Lumbricidae in the rhizosphere of Hordelymus europaeus (Poaceae): faunal interactions, response of microorganisms and effects on plant growth. Oecologia 106, 111–126.
Anderson J M and Ineson P 1984 Interactions between microorganisms and soil invertebrates in nutrient flux pathways of forest ecosystems. In Invertebrate-microbial interactions. Eds. J M Anderson, A D M Rayner and D W H Walton. pp 59–88. Cambridge University Press, Cambridge.
Bååth E, Lohm U, Lundgren B, Rosswall T, Södeström B and Sohlenius B 1981 Impact of microbial feeding animals on total soil activity and nitrogen dynamics: a soil microcosm experiment. Oikos 37, 257–264.
Begon M, Harper J L and Townsend C R 1990 Ecology: individuals, populations and communities. Blackwell Scientific Publications, London.
Bengtsson J, Setälä H and Zheng D W 1996 Food webs and nutrient cycling in soils: interactions and positive feedbacks. In Food webs: integration of patterns and dynamics. Eds. G A Polis and K O Winemiller. pp 30–38. Chapman & Hall, New York.
Bowman L A, Bloem J, van den Boogert P H J F, Bremer F, Hoenderboom G H J and de Ruiter P C 1994 Short-term and long-term effects of bacterivorous nematodes and nematophagous fungi on carbon and nitrogen mineralisation in microcosms. Biol. Fertil. Soils 17, 249–256.
Breland T A and Bakken R L 1991 Microbial growth and nitrogen immobilization in the root zone of barley (Hordeum vulgare L.), Italian ryegrass (Lolium multiflorum Lam.), and white clover (Trifolium repens L.). Biol. Fertil. Soils 12, 154–160.
Carpenter S R, Kitchell J F and Hodgson J R 1985 Cascading trophic interactions and lake productivity. BioScience 35, 634–639.
DeAngelis D L 1992 Dynamics of nutrient cycling and food webs, 1st edition. Chapman and Hall, London.
Didden W A M 1993 Ecology of terrestrial Enchytraeidae. Pedobiologia 37, 2–29.
Elliott E T, Coleman D C and Cole C V 1979 The influence of amoebae on the uptake of nitrogen by plants in gnotobiotic soil. In The soil-root interface. Eds. J L Harley and R S Russell. pp 221–229. Academic Press, New York.
Faber J H and Verhoef H A 1991 Functional differences between closely-related soil arthropods with respect to decomposition processes in the presence or absence of pine tree roots. Soil Biol. Biochem. 23, 15–23.
Frostegå rd Å, Tunlid A and Bååth E 1993 Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl. Environ. Microbiol. 59, 3605–3617.
Frostegå rd Å and Bååth E 1996 The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 22, 59–65.
Haimi J and Einbork M 1992 Effects of endogeic earthworms on soil processes and plant growth in coniferous forest soil. Biol. Fertil. Soils 13, 6–10.
Hanlon R D G 1981 Influence of grazing by Collembola on the activity of senescent fungal colonies grown on media of different nutrient concentration. Oikos 36, 362–367.
Hanlon R D G and Anderson J M 1979 The effects of collembola grazing on microbial activity in decomposing leaf litter. Oecologia 38, 93–99.
Hanlon R D G and Anderson J M 1980 The influence of macroarthropod feeding activities on microflora in decomposing oak leaves. Soil Biol. Biochem. 12, 255–261.
Hunt H W, Coleman D C, Ingham E R, Ingham R E, Elliott E T, Moore J C, Rose S L, Reid C P P and Morley C R 1987 The detrital food web in a shortgrass prairie. Biol. Fertil. Soils 3, 57–68.
Ingham R E, Trofymow J A, Ingham E R and Coleman D C 1985 Interactions of bacteria, fungi, and their nematode grazers: effects on nutrient cycling and plant growth. Ecol. Monogr. 55, 119–140.
Jenkinson D S 1988 Determination of microbial biomass carbon and nitrogen in soil. In Advances in nitrogen cycling in agricultural ecosystems. Ed. J R Wilson. pp 368–386. Proceedings of the Symposium on Advances in Nitrogen Cycling in Agricultural Ecosystems held in Brisbane, Australia, 11- 15th May 1987.
Karg W 1986 Acari (Acarina), Milben Unterordnung Anactinochaeta (Parasitiformes) Die freilebenden Gamasina (Gamasides), Raubmilben. Die Tierwelt Deutschlands. Teil 59. VEB Gustav Fisher Verlag, Jena. 475 p.
Laakso J, Salminen J and Setälä H 1995 Effects of abiotic conditions and microarthropod predation on the structure and function of soil animal communities. Acta Zoologica Fennica 196, 162–167.
Laakso J and Setälä H 1999a Population and ecosystem-level effects of predation on nematodes. Oecologia 120, 279–286.
Laakso J and Setälä H 1999b Sensitivity of primary production to changes in the architecture of belowground food webs. Oikos 87, 57–64
Lawton J H 1994 What do species do in ecosystems? Oikos 71, 367–374.
Leibold M A and Wilbur H M 1992 Interactions between food-web structure and nutrients on pond organisms. Nature 360, 341–343.
Martikainen E and Huhta V 1990 Interactions between nematodes and predatory mites in raw humus soil: a microcosm experiment. Revue D'Ecologie et de Biologie du Sol 27, 13–20.
McGrady-Steed J, Harris P M and Morin P 1997 Biodiversity regulates ecosystem predictability. Nature 390, 162–165.
McNaughton S J, Oesterheld M, Frank D A and Williams K J 1989 Ecosystem-level patterns of primary productivity and herbivory in terrestrial habitats. Nature 341, 142–144.
Mikola J and Setälä H 1998a No evidence of trophic cascades in an experimental microbial-based soil food web. Ecology 79, 153–164.
Mikola J and Setälä H 1998b Relating species diversity to ecosystem functioning-mechanistic backgrounds and experimental approach with a decomposer food web. Oikos 83, 180–194.
Mikola J and Setälä H 1998c Productivity and trophic level biomasses in a microbial-based soil food web. Oikos 82, 158–168.
Moore J C, Walter DE and Hunt H W 1988 Arthropod regulation of micro-and mesobiota in below-ground detrital food webs. Annu. Rev. Entomol. 33, 419–439.
Nylund J E and Wallander H 1992 Ergosterol analysis as means of quantifying mychorrhizal biomass. In Methods in microbiology. Eds. J R Norris, D J Read and A K Varma. Vol. 24. pp 77–88. Academic Press, London.
Oksanen L, Fretwell S, Arruda J and Niemelä P 1981 Exploitation ecosystems in gradients of primary productivity. American Naturalist 118, 240–261.
Paine G G 1966 Food web complexity and species diversity. American Naturalist 100, 65–75.
Palojärvi A, Sharma S, Rangger A, von Luetzow M and Insam H 1997 Comparison of Biolog and phospholipid fatty acid patterns to detect changes in microbial community. In Microbial communities.Functional versus structural approaches. Eds. H Insam and A Rangger. pp 37–48. Springer Verlag, Berlin.
Pimm S 1982 Food webs. Chapman and Hall, London.
Polis G A and Strong D R 1996 Food web complexity and community dynamics. American Naturalist 147, 813–846.
Ponge J F 1991 Food resources and diets of soil animals in small area of Scots pine litter. Geoderma 49, 33–62.
Power M E 1992 Top-down and bottom-up forces in food webs: do plants have primacy? Ecology 73, 733–746.
de Ruiter P C, Neutel A-M and Moore J C 1995 Energetics, patterns of interaction strengths, and stability in real ecosystems. Science 269, 1257–1260.
Setälä H, Tyynismaa M, Martikainen E and Huhta V 1991 Mineralisation of C, N and P in relation to decomposer community structure in coniferous forest soil. Pedobiologia 35, 285–296.
Setälä H, Marshall V G and Trofymov J A 1997 Influence of body size of soil fauna on litter decomposition and 15N uptake by poplar in a pot trial. Soil Biol. Biochem. 28, 1661–1675.
Siepel H and de Ruiter-Dijkman E M 1993 Feeding quilds of oribatid mites based on their carbohydrase activities. Soil Biol. Biochem. 25, 1491-1497.
Sohlenius B 1969 The monoxenic cultivation of some Rhabditid nematodes. Oikos 20, 287–293.
Sulkava P, Huhta V and Laakso J 1996 Impact of soil faunal structure on decomposition and N-mineralisation in relation to temperature and moisture in forest soil. Pedobiologia 40, 505–513.
Swift M J, Heal O J and Anderson J M 1979 Decomposition in terrestrial ecosystems. Blackwell, Oxford.
Vance E D, Brookes P C and Jenkinson D S 1987 An extraction method for measuring microbial biomass. Soil Biol. Biochem. 19, 703–707.
Verhoef H A and Brussaard L 1990 Decomposition and nitrogen mineralization in natural and agroecosystems: the contribution of soil animals. Biogeochemistry 11, 175–211.
Visser S 1985 Role of soil invertebrates in determining the composition of soil microbial communities. In Ecological interactions in soil; plants, microbes and animals. Eds. A H Fitter, D Atkinson, D J Read and M B Usher. 2nd edition. pp 297–317. Blackwell Scientific Publications, Oxford.
Wardle D A 1992 A comparative assesment of factors which influence microbial Biomass carbon and nitrogen levels in soil. Biol. Rev. 67, 321–358.
Wardle D A and Yeates G W 1993 The dual importance of competition and predation as regulatory forces in terrestrial ecosystems: evidence from decomposer food-webs. Oecologia 93, 303–306.
Wardle D A, Zackrisson O, Hörnberg G and Gallet C 1997 The influence of island area on ecosystem properties. Science 277, 1296–1299.
Williams S T and Gray T R G 1974 Decomposition of litter on the soil surface. In Biology of plant litter decomposition. Eds. C H Dickinson and G J F Pugh. pp 611–631.
Wu J, Joergensen R G, Pommerening B, Chaussod R and Brookes P C 1990 Measurement of soil microbial biomass C by fumigationextraction-an automated procedure. Soil Biology and Biochemistry 22, 1167–1169.
Yeates G W, Bongers T, de Goede R G M, Freckman D W and Georgiewa S S 1993 Feeding habits in soil nematode families and genera-an outline for soil ecologists. Journal Of Nematology 25, 315–329.
Zelles L, Bai Q Y, Beck T and Beese F 1992 Signature fatty acids in phospholipids and lipopolysaccharides as indicators of microbial biomass and community structure in agricultural soils. Soil Biol. Biochem. 24, 317–323.
Zheng D W, Bengtsson J and Ågren G I 1997 Soil food webs and ecosystem processes: decomposition in donor-control and Lotka-Volterra systems. American Naturalist 149, 125–148.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Laakso, J., Setälä, H. & Palojärvi, A. Influence of decomposer food web structure and nitrogen availability on plant growth. Plant and Soil 225, 153–165 (2000). https://doi.org/10.1023/A:1026534812422
Issue Date:
DOI: https://doi.org/10.1023/A:1026534812422