Abstract
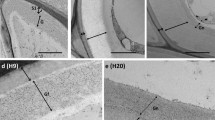
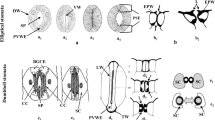
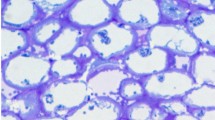
Using a silver-enhanced, gold-secondary antibody immuno-location approach, we investigated the mechanisms for the switch from β-1,4- to β-1,3-glucan biosynthesis upon wounding. Antibodies against β-1,4- and β-1,3-glucan synthases were used to locate these synthases before and after wounding of Mung bean (Vigna radiata var Berken) hypocotyls. Within 5 min of wounding, β-1,4-glucan synthases which were densely localized on plasma membranes adjacent to the secondary walls at the wound site completely disappeared, and β-1,3-glucan synthases became labeled. The immuno-location of the β-1,3-glucan synthases in the secondary walls was in good accordance with the region where the β-1,4-glucan synthases were localized before wounding. Aniline blue was also utilized to visualize the deposition of callose upon wounding. Within 5 min of wounding, callose had accumulated in the corresponding region where the immuno-labeling of β-1,3-glucan synthase was detected after wounding. The β-1,3-glucan synthases were always detected from the sieve plate and plasmodesmata which are known to have constitutive synthesis of callose regardless of wounding. Secondary walls located distantly into the tissue away from the wound site were consistently labeled by the β-1,4-glucan synthase antibody even after wounding. Immuno-blot analysis clearly shows that the levels of β-1,4-glucan synthase subunit Ces A decreased dramatically within 30 min, whereas the β-1,3-glucan synthase subunit CFL1 levels increased significantly after wounding. The intensity of labeling reached a maximum at the wound site, and gradually decreased in correspondence with the distance from the wound site. When a protease inhibitor cocktail was applied upon wounding, neither the β-1,3-glucan synthase appeared nor callose was deposited during the first 5 min of wounding. On the other hand, β-1,4-glucan synthase was detected at the wound site, implying that activation of β-1,3-glucan synthase may rely on the degradation of the β-1,4-glucan synthase. Our study may provide new insight into β-glucan synthesis in higher plants.
Similar content being viewed by others
References
Amor Y., Haigler C. H., Wainscott M., Johnson S. and Delmer D. P. 1995. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc. Natl Acad. Sci. USA 92: 9353–9357.
Bradford M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 72: 248–254.
Brown R. M.Jr. and Montezinos D. 1976. Cellulose microfibrils: visualization of biosynthetic and oriented complexes in association with the plasma membrane. Proc. Natl Acad. Sci. USA 73: 143–147.
Brown R. M. Jr. 1996. The biosynthesis of cellulose. J. Macromol. Sci. A33(10): 1345–1373.
Carpita N. C. and Delmer D. P. 1980. Protection of cellulose synthesis in detached cotton fibers by polyethylene glycol. Plant Physiol. 66: 911–916.
Chao W. S., Gu Y. Q., Pautot V., Bray E. A. and Walling L. L. 1999. Leucine aminopeptidase RNAs, proteins, and activities increase in response to water deficit, salinity, and the wound signals systemin, methyl jasmonate, and abscisic acid. Plant Physiol. 120: 979–992.
Cui X., Shin H., Song C., Laosinchai W., Amano Y. and Brown R. M. Jr. 2001. A putative plant homolog of the yeast β-1, 3-glucan synthase subunit FKS1 from cotton (Gossypium hirsutum L. ) fibers. Planta 213: 223–230.
Delmer D. P. 1983. Biosynthesis of cellulose. Adv. Carbohydr. Chem. Biochem. 41: 105–153.
Delmer D. P. 1987. Cellulose biosynthesis. Annu. Rev. Plant Physiol. 38: 259–290.
Delmer D. P. 1999. Cellulose synthesis: exciting times for a difficult field of study. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50: 245–276.
Doblin M. S., De Melis L., Newbigin E., Bacic A. and Read S. M. 2001. Pollen tubes of Nicotiana alata express two genes from different β-glucan synthase families. Plant Physiol. 125: 2040–2052.
Douglas C. M., Foor F. and Marrinan J. A. 1994. The sacchar-omyces cerevisiae FKS1 (ETG1) gene encodes an integral membrane protein which is a subunit of 1, 3-β-D-glucan synthase. Proc. Natl Acad. Sci. USA 91: 12907–129011.
Farmer E. E. and Ryan C. A. 1990. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl Acad. Sci. USA 87: 7713–7716.
Farmer E. E. and Ryan C. A. 1992. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible pro-teinase inhibitors. The Plant Cell 4: 129–134.
Ferguson C., Teeri T. T., Siika-aho M., Read S. M. and Bacic A. 1998. Location of cellulose and callose in pollen tubes and grains of Nicotiana tabacum. Planta 206: 452–460.
Fincher G. B. and Stone B. A. 1981. Metabolism of noncellulosic polysaccharides. In: Tanner W. and Loewus F. A. (eds), Encyclopedia of Plant Physiology, New Series 13B, Springer-Verlag, Berlin, Germany, pp. 68–132.
Franz G. and Blaschek W. 1990. Cellulose. Meth. Plant Biochem. 2: 291–322.
Giddings T. H., Brower D. L. and Staehelin L. A. 1980. Visualization of particle complexes in the plasma membrane of Micrasterias denticulata associated with the formation of cellulose fibrils. J. Cell Biol. 84: 327–339.
Girard L. and MacLachlan G. A. 1985. ''Modulation of Pea Membrane''-Glucan synthase activity by calcium, polyca-tion, endogenous protease, and protease inhibitors. Plant Physiol. 85: 131–136.
Hermann S. and Gebhard V. J. 1987. Tricine-SDS Poly Acrylamide Gel Electrophoresis for the separation of proteins in the range from1 to 100 kDa. Analyt. Biochem. 166: 368–379.
Hong Z., Delauney A. J. and Verma D. P. 2001. A cell plate-specific callose synthase and its interaction with phragmoplas-tin. The Plant Cell 13: 755–768.
Hotchkiss A. T. Jr. and Brown R. M. Jr. 1987. The association of rosette and globule terminal complexes with cellulose microfibril assembly in Nitella translucens (Charophyceae). J. Phycol. 23: 229–237.
Jacob S. R. and Northcote D. H. 1985. In vitro glucan synthesis by membranes of celery petioles: the role of the membrane in deter-mining the type of linkage formed. J. Cell Sci. 2(Suppl. ): 1–11.
Kauss H., Kohle H. and Jeblick W. 1983. Proteolytic activation and stimulation by Ca 2+ of glucan synthase from soybean cells. FEBS Lett. 158: 84–88.
Kimura S. and Itoh T. 1995. Evidence for the role of the glomerulocyte in cellulose synthesis in the tunicate, Metandrocarpa uedai. Protoplasma 186: 24–33.
Kimura S., Laosinchai W., Itoh T., Cui X., Linder R. and Brown R. M. Jr. 1999. Immunogold labeling of rosette term-inal cellulose-synthesizing complexes in the vascular plant Vigna angularis. The Plant Cell 11: 2075–2085.
Kudlicka K. and Brown R. M. Jr. 1997. Cellulose and callose biosynthesis in higher plants. 1. Solubilization and separation of (1! 3)-and (1!4)-β-glucan synthase activities from mung bean. Plant Physiol. 115: 643–656.
Mueller S. C. and Brown R. M. Jr. 1980. Evidence for an intra-membrane component associated with a cellulose microfibril synthesizing complex in higher plants. J. Cell Biol. 84: 315–326.
Nakashima J., Awano T., Takabe K., Fujita M. and Saiki H. 1997. Immunocytochemical localization of phenylalanine ammonia-lyase and cinnamyl alcohol dehydrogenase in differentiating tracheary elements derived from Zinnia meso-phyll cells. Plant Cell Physiol. 38: 113–123.
Narvaez-Vasquez J., Pearce G., Orozco-Cardenas M. L., Franceschi V. R. and Ryan C. A. 1995. Autoradiographic and biochemical evidence for the systemic translocation of systemin in tomato plants. Planta 195: 593–600.
Pear J., Kawagoe Y., Schreckengost W., Delmer D. P. and Stalker D. 1996. Higher plants contain homologs of the bac-terial CelA genes encoding the catalytic subunit of the cellulose synthase. Proc. Natl Acad. Sci. USA 93: 12637–12642.
Pearce G., Strydom D., Johnson S. and Ryan C. A. 1991. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 253: 895–898.
Pena-Cortes H., Fisahn J. and Willmitzer L. 1995. Signals involved in wound-induced proteinase inhibitor II gene expression in tomato and potato plants. Proc. Natl Acad. Sci. USA 92: 4106–4113.
Rong F. and Brown R. M. Jr. 2000. A novel cotton ovule culture: induction, growth, and characterization of submerged cotton fibers (Gossypium hirsutum L. ). In Vitro Cell. Devel. Biol.-Plant 36: 293–299.
Saxena I. M., Kudlicka K., Okuda K. and Brown R. M. Jr. 1994. Characterization of genes in the cellulose-synthesizing operon (acs operon) of Acetobacter xylinum: implications for cellulose crystallization. J. Bacteriol. 176: 5735–5752.
Saxena I. M., Brown R. M. Jr., Fevre M., Geremia R. A. and Henrissat B. 1995. Multidomain architecture of β-glycosyl transferases: implications for mechanism of action. J. Bacteriol. 177: 1419–1424.
Schnepf E., White O., Rudolph U., Deichgraber G. and Reiss H. D. 1985. Tip cell growth and the frequency and distribu-tion of particle rosettes in the plasmalemma: experimental studies of in Funaria protonema cells. Protoplasma 127: 222–229.
Silber K. R., Keiler K. C. and Sauer R. T. 1992. Tsp: a tail-specific protease that selectively degrades proteins with nonpolar C termini. Proc. Natl Acad. Sci. USA 89: 295–299.
Stone B. A. and Clarke A. E. 1992. Chemistry and Biology of (1!3)-β-Glucans. La Trobe University Press, Bendigo, Australia, pp. 365–429.
Verma D. P. 2001. Cytokinesis and building of the cell plate in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52: 751–784.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakashima, J., Laosinchai, W., Cui, X. et al. New insight into the mechanism of cellulose and callose biosynthesis: proteases may regulate callose biosynthesis upon wounding. Cellulose 10, 369–389 (2003). https://doi.org/10.1023/A:1027336605479
Issue Date:
DOI: https://doi.org/10.1023/A:1027336605479