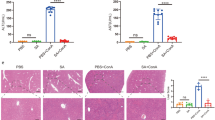
Abstract
Multiple lines of evidence suggest a role for endogenous lipopolysaccharides in toxin-induced liver injury. Toll-like receptor 4 has recently been implicated as a cell surface receptor important for lipopolysaccharide responsiveness. In these experiments, we sought to determine the role of toll-like receptor 4 in acute liver injury by carbon tetrachloride by utilizing the naturally occurring toll-like receptor 4 mutant and wild-type mice strains. Mice were injected with either carbon tetrachloride or the carrier. Serum transaminase levels peaked at 24 hr after carbon tetrachloride administration for both wild-type and mutant mice, with no significant histological difference in initial liver injury between the two groups. However, an overall decrease in hepatocyte proliferation was found in the mutant mice. Examination of the liver tissue revealed significant decreases in intrahepatic expressions of proinflammatory mediators. In conclusion, our results suggest that toll-like receptor 4 is important in the hepatic regenerative response to CCl4 liver injury via its role in modulating the inflammatory response to hepatic injury.
Similar content being viewed by others
REFERENCES
Nolan JP: The role of endotoxin in liver injury. Gastroenterology 69:1346–1356, 1975
Nolan JP: Intestinal endotoxins as mediators of hepatic injury-an idea whose time has come again. Hepatology 10:887–891, 1989
Roth RA, Harkema JR, Pestka JP, Ganey PE: Is exposure to bacterial endotoxin a determinant of susceptibility to intoxication from xenobiotic agents? Toxicol Appl Pharmacol 147:300–311, 1997
Nolan JP, Leibowitz AI: Endotoxin and the liver III. Modification of acute carbon tetrachloride injury by polymyxin B, an antiendotoxin. Gastroenterology 75:445–449, 1978
Williams J: Carbon tetrachloride hepatotoxicity in endotoxin tolerant and polymixin B treated rats. Int J Immunopharm 10:975–980, 1988
Nolan JP, Ali MV: Endotoxin and the liver: II. Effect of tolerance on carbon tetrachloride-induced liver injury. J Med 4:28–38, 1973
Chamulitrat W, Jordan S, Mason R: Nitric oxide production during endotoxin shock in carbon tetachloride-treated rats. Mol Pharm 46:391–397, 1994
Czaja MJ, Xu J, Ju J, Alt E, Schmiedeberg P: Lipopolysaccharide-neutralizing antibody reduces hepatocyte injury from acute hepatotoxin administration. Hepatology 19:1282–1289, 1994
Cornell RP, Liljequist BL, Bartizal KF: Depressed liver regeneration after partial hepatectomy of germ-free, athymic and lipopolysaccharide-resistant mice. Hepatology 11:916–922, 1990
Edwards MJ, Keller BJ, Kauffman FC, Thurman RG: The involvement of Kupffer cells in carbon tetrachloride toxicity. Toxicol Appl Pharmacol 119:275–279, 1993
Olthoff, K.M. (2002) Molecular Pathways of Regeneration and Repair after Liver Transplantation. World J Surg 26.
Su GL, Goyert SM, Fan MH, Aminlari A, Gong KO, Klein RD, Myc A, Alarcon WH, Steinstraesser L, Remick, DG, Wang SC: Activation of human and mouse Kupffer cells by lipopolysaccharide is mediated by CD14. Am J Physiol Gastrointest Liver Physiol 283:G640–G645, 2002
Su G, Klein R, Aminlari A, Zhang H, Steinstraesser L, Alarcon W, Remick D, Wang S: Kupffer cell activation by lipopolysaccharide in rats: role for lipopolysaccharide binding protein and toll-like receptor 4. Hepatology 31:932–936, 2000
Akira S: Mammalian Toll-like receptors. Curr Opin Immunol 15:5–11, 2003
Poltorak A, He X, Smirnova I, Liu M, Van Huffel C, Du X, Birdwell D, Alejos E., Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B: Defective LPS signaling in C3H/HeJ and C57BL/10ScCr Mice: mutations in Tlr4 gene. Science 282:2085–2088, 1998
Cattoretti G, Pileri S, Parravicini C, Becker MH, Poggi S, Bifulco C, Key G, D'Amato L, Sabattini E, Feudale E, et al.: Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol 171:83–98, 1993
Shi SR, Imam SA, Young L, Cote RJ, Taylor CR: Antigen retrieval immunohistochemistry under the influence of pH using monoclonal antibodies. J Histochem Cytochem 43:193–201, 1995
Su GL, Freeswick PD, Geller DA, Wang Q, Shapiro RA, Wan YH, Billiar TR, Tweardy DJ, Simmons RL, Wang SC: Mollcular cloning, characterization, and tissue distribution of rat lipopolysaccharide binding protein. Evidence for extrahepatic expression. J Immunol 153:743–752, 1994
Petroll WM, Jester JV, Bean J, Cavanagh HD: Labeling of cycling corneal endothelial cells during healing with a monoclonal antibody to the Ki67 antigen (MIB-1). Cornea 18:98–108, 1999
Farinati F, Cardin R, D'Errico A, De Maria N, Naccarato R, Cecchetto A, Grigioni W: Hepatocyte proliferative activity in chronic liver damage as assessed by the monoclonal antibody MIB1 Ki67 in archival material: The role of etiology, disease activity, iron, and lipid peroxidation. Hepatology 23:1468–1475, 1996
Fausto N: Liver regeneration. J Hepatol 32:19–31, 2000
Rai RM, Loffreda S, Karp CL, Yang SQ, Lin HZ, Diehl AM: Kupffer cell depletion abolishes induction of interleukin-10 and permits sustained overexpression of tumor necrosis factor alpha messenger RNA in the regenerating rat liver. Hepatology 25:889–895, 1997
Casado M, Callejas NA, Rodrigo J, Zhao X, Dey SK, Bosca L, Martin-Sanz P: Contribution of cyclooxygenase 2 to liver regeneration after partial hepatectomy. FASEB J 15:2016–2018, 2001
Czaja MJ, Xu J, Alt E: Prevention of carbon tetrachloride-induced rat liver injury by soluble tumor necrosis factor receptor. Gastroenterology 108:1849–1854, 1995
Bruccoleri A, Gallucci R, Germolec D, Blackshear P, Simeonova P, Thurman R, Luster M: Induction of early-immediate genes by tumor necrosis factor ? contribute to liver repair following chemical induced hepatotoxicity. Hepatology 25:133–141, 1997
Yamada Y, Fausto N: Deficient liver regeneration after carbon tetrachloride injury in mice lacking type 1 but not type 2 tumor necrosis factor receptor. Am J Pathol 152:1577–1589, 1998
Grisham JW, Coleman WB: Molecular regulation of hepatocyte generation in adult animals. Am J Pathol 161:1107–1110, 2002
Streetz KL, Luedde T, Manns MP, Trautwein C: Interleukin 6 and liver regeneration. Gut 47:309–312, 2000
Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R: Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274:1379–1383, 1996
Tjalve H, Lofberg B: Extrahepatic sites of metabolism of carbon tetrachloride in rats. Chem Biol Interact 46:299–316, 1983
Smith WL, Langenbach R: Why there are two cyclooxygenase isozymes. J Clin Invest 107:1491–1495, 2001
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Su, G.L., Wang, S.C., Aminlari, A. et al. Impaired Hepatocyte Regeneration in Toll-Like Receptor 4 Mutant Mice. Dig Dis Sci 49, 843–849 (2004). https://doi.org/10.1023/B:DDAS.0000030097.52476.aa
Issue Date:
DOI: https://doi.org/10.1023/B:DDAS.0000030097.52476.aa