Abstract
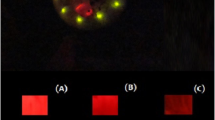
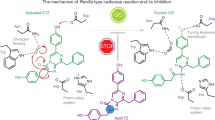
Beetle luciferases, the enzymes responsible for bioluminescence, are special cases of CoA-ligases which have acquired a novel oxygenase activity, offering elegant models to investigate the structural origin of novel catalytic functions in enzymes. What the original function of their ancestors was, and how the new oxygenase function emerged leading to bioluminescence remains unclear. To address these questions, we solved the crystal structure of a recently cloned Malpighian luciferase-like enzyme of unknown function from Zophobas morio mealworms, which displays weak luminescence with ATP and the xenobiotic firefly d-luciferin. The three dimensional structure of the N-terminal domain showed the expected general fold of CoA-ligases, with a unique carboxylic substrate binding pocket, permitting the binding and CoA-thioesterification activity with a broad range of carboxylic substrates, including short-, medium-chain and aromatic acids, indicating a generalist function consistent with a xenobiotic-ligase. The thioesterification activity with l-luciferin, but not with the d-enantiomer, confirms that the oxygenase activity emerged from a stereoselective impediment of the thioesterification reaction with the latter, favoring the alternative chemiluminescence oxidative reaction. The structure and site-directed mutagenesis support the involvement of the main-chain amide carbonyl of the invariant glycine G323 as the catalytic base for luciferin C4 proton abstraction during the oxygenase activity in this enzyme and in beetle luciferases (G343).
Similar content being viewed by others
Abbreviations
- Zmo:
-
Zophobas morio
- Ppy:
-
Photinus pyralis
- Lcr:
-
Luciola cruciata.
References
T. Wilson and J. W. Hastings, Bioluminescence, Annu. Rev. Cell Dev. Biol., 1998, 14, 197–230.
H. H. Seliger, The origin of bioluminescence, Photochem. Photobiol., 1975, 21, 355–361.
H. H. Seliger, The evolution of bioluminescence in bacteria, Photochem. Photobiol., 1987, 45, 291–297.
J. F. Rees, B. Wergifosse, O. Noiset, M. Dubuisson, B. Janssens and E. M. Thompson, The origins of marine bioluminescence: turning oxygen defense mechanisms into deep-sea communication tools, J. Exp. Biol., 1998, 201, 1211–1221.
J. W. Hastings, Bioluminescence, in Cell Physiology, ed. N. Sperelakis, Academic Press, New York, 2001, pp. 1115–1131.
V. R. Viviani, The origin, diversity and structure function relationships of insects luciferases, Cell. Mol. Life Sci., 2002, 59, 1833–1850.
K. V. Wood, The chemical mechanism and evolutionary development of beetle bioluminescence, Photochem. Photobiol., 1995, 62, 662–673.
V. R. Viviani and J. H. Bechara, Larval Tenebrio molitor (Coleoptera: Tenebrionidae) fat body extracts catalyze D-luciferin and ATP-dependent chemiluminescence. A luciferase-like enzyme, Photochem. Photobiol., 1996, 63, 713–718.
V. R. Viviani, R. A. Prado, F. G. C. Arnoldi and F. C. Abdalla, An ancestral luciferase in the Malpighian tubules of a non-bioluminescent beetle, Photochem. Photobiol. Sci., 2009, 8, 57–61.
V. R. Viviani, V. Scorsato, R. A. Prado, J. G. C. Pereira, K. Niwa, Y. Ohmiya and J. A. R. G. Barbosa, The origin of luciferase activity in Zophobas mealworm AMP/CoA-ligase (protoluciferase): luciferin stereoselectivity as a switch for the oxygenase activity, Photochem. Photobiol. Sci., 2010, 9, 1111–1119.
V. R. Viviani, R. A. Prado, D. R. Neves, D. Kato and J. A. Barbosa, A route from darkness to light: emergence and evolution of luciferase activity in AMP-CoA-ligases inferred from a mealworm luciferase-like enzyme, Biochemistry, 2013, 52, 3963–3973.
R. A. Prado, J. A. Barbosa, Y. Ohmiya and V. R. Viviani, Structural evolution of luciferase activity in Zophobas, mealworm AMP/CoA-ligase (protoluciferase) through site-directed mutagenesis of the luciferin binding site, Photochem. Photobiol. Sci., 2011, 10, 1226–1232.
J. A. McCoy, R. W. Grosse-Kunstleve, P. D. Adams, M. D. Winn, L. C. Storoni and R. J. Read, Phaser crystallographic software, J. Appl. Crystallogr., 2007, 40, 658–674.
T. C. Terwiller, R. W. Grosse-Kunstleve, P. V. Afonine, N. W. Moriarty, P. H. Zwart, L. W. Hung, R. J. Read and P. D. Adams, Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2008, 64, 61–69.
N. Echols, R. W. Grosse-Kunstleve, P. V. Afonine, G. Bunkóczi, V. B. Chen, J. J. Headd, J. A. McCoy, N. W. Moriarty, R. J. Read, D. C. Richardson, J. S. Richardson, T. C. Terwilliger and P. D. Adams, Graphical tools for macromolecular crystallography in PHENIX, J. Appl. Crystallogr., 2012, 45, 581–586.
P. V. Afonine, R. W. Grosse-Kunstleve, N. Echols, J. J. Headd, N. W. Moriarty, M. Mustyakimov, T. C. Terwilliger, A. Urzhumtsev, P. H. Zwart and P. D. Adams, Towards automated crystallographic structure refinement with phenix.refine, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2012, 68, 352–367.
P. Emsley, B. Lohkamp, W. G. Scott and K. Cowtan, Features and development of Coot, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2010, 66, 486–501.
V. B. Chen, W. B. Arendall 3rd, J. J. Headd, D. A. Keedy, R. M. Immormino, G. J. Kapral, L. W. Murray, J. S. Richardson and D. C. Richardson, MolProbity: all-atom structure validation for macromolecular crystallography, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2010, 66, 12–21.
V. R. Viviani and Y. Ohmiya, Bovine serum albumin displays luciferase-like activity in presence of luciferyl-adenylate: insights on the origin of protoluciferase activity and bioluminescence colours, Luminescence, 2006, 21, 262–267.
D. Kato, Y. Hiraishi, M. Maenaka, K. Yokoyama, K. Niwa, Y. Ohmiya, M. Takeo and S. Negoro, Interconversion of ketoprofen recognition in firefly luciferase-catalyzed enantioselective thioesterification reaction using from Pylocoeria miyako (PmL) and Hotaria parvura (HpL) just by mutating two amino acid residues, J. Biotechnol., 2013, 168, 277–283.
E. Conti, N. P. Franks and P. Brick, Crystal structure of firefly luciferase throws light on a superfamily of adenylate-forming enzymes, Structure, 1996, 4, 287–298.
T. Nakatsu, S. Ichiyama, J. Hiratale, A. Saldanha, N. Kibashi, K. Sakata and H. Kato, Structural basis for the spectral difference in luciferase bioluminescence, Nature, 2006, 440, 372–376.
A. M. Gulick, V. J. Starai, A. R. Horswill, K. M. Homick and J. C. Escalante-Semerena, The 1,75 Å crystal structure of acetyl-CoA synthetase bound to adenosine-5′-propylphosphate and coenzyme A, Biochemistry, 2003, 42, 2866–2873.
T. Zako, K. Ayabe, T. Aburatani, N. Kamiya, A. Kitayama, H. Ueda and T. Nagamune, Luminescent and substrate binding activities of firefly luciferase N-terminal domain, Biochim. Biophys. Acta, 2003, 1649, 183–189.
B. R. Branchini, R. A. Magyar, M. H. Murtiashaw, S. M. Anderson and V. Zimmer, Site-directed mutagenesis of histidine 245 in firefly luciferase: a proposed model of the active site, Biochemistry, 1998, 37, 15311–15319.
B. R. Branchini, R. A. Magyar, M. H. Murtiashaw, S. M. Anderson, L. C. Helgerson and M. Zimmer, Site-directed mutagenesis of firefly luciferase active site amino acids: a proposed model for bioluminescence color, Biochemistry, 1999, 38, 13223–13230.
M. Knights and C. J. Drogemuller, Xenobiotic-CoA ligases: kinetic and molecular characterization, Curr. Drug Metab., 2000, 1, 49–66.
H. P. Stuible and E. Kombrink, Identification of the substrate specificity-conferring amino-acid residues of 4-coumarate: CoenzymeA ligase allows the rational design of mutant enzymes with new catalytic properties, J. Biol. Chem., 2001, 276, 26893–26897.
T. Beuerle and E. Pichersky, Purification and characterization of benzoate: coenzyme A ligase from Clarkia breweri, Arch. Biochem. Biophys., 2002, 400, 258–264.
Y. Oba, M. Ojika and S. Inouye, Firefly luciferase is a bifunctional enzyme: ATP-dependent monooxygenase and a long chain fatty acyl-CoA synthetase, FEBS Lett., 2003, 540, 251–254.
W. C. Rhodes and W. D. McElroy, The synthesis and function of luciferyl-adenylatwe and oxyluciferyl-adenylate, J. Biol. Chem., 1958, 233, 1528–1537.
E. H. White, E. Rapaport, H. H. Seliger and T. A. Hopkins, The chemi- and bioluminescence of firefly luciferin: an efficient chemical production of electronically excited states, Bioorg. Chem., 1971, 1, 92–122.
B. R. Branchini, T. L. Southworth, M. H. Murtiashaw, H. Boije and S. E. Fleet, A mutagenesis study of the putative luciferin binding site residues of firefly luciferase, Biochemistry, 2003, 42, 10429–10436.
B. R. Branchini, T. L. Southworth, M. H. Murtiashaw, S. R. Wilkinson, N. F. Khattak, J. C. Rosenberg and M. Zimmer, Mutagenesis evidence that the partial reactions of firefly bioluminescence are catalyzed by different conformations of the luciferase C-terminal domain, Biochemistry, 2005, 44, 1385–1393.
B. R. Branchini, C. E. Behney, T. L. Southworth, D. M. Fontaine, A. M. Gulick, D. J. Vinyard and G. W. Brudvig, Experimental Support for a Single Electron-Transfer Oxidation Mechanism in Firefly Bioluminescence, Am. Chem. Soc., 2015, 137(24), 7592–7595.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Prado, R.A., Santos, C.R., Kato, D.I. et al. The dark and bright sides of an enzyme: a three dimensional structure of the N-terminal domain of Zophobas morio luciferase-like enzyme, inferences on the biological function and origin of oxygenase/luciferase activity. Photochem Photobiol Sci 15, 654–665 (2016). https://doi.org/10.1039/c6pp00017g
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c6pp00017g