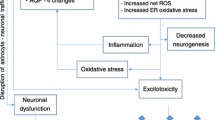
Abstract
Thiamine or vitamin B1, an essential nutrient absorbed from the diet, is involved in vital brain metabolic and cellular functions, including carbohydrate metabolism and neurotransmitter production. Diencephalic regions and, in particular, the cerebellum demonstrate lesions in cases of prolonged thiamine deficiency, such as that observed in alcohol-dependent individuals or in patients with cancer or AIDS. The purpose of this review is to demonstrate recent evidence of cerebellar dysfunction resulting from thiamine deficiency and to assemble theories as to why the cerebellum may be sensitive to this type of insult. A brief outline on cerebellar structure and function, as well as a short discussion on thiamine and thiamine deficiency are provided before detailing the conditions and mechanisms underlying thiamine deficiency-induced cerebellar dysfunction. Although much is known regarding cell loss from a lack of thiamine, further work is still required to identify the sequelae of events leading to the susceptibility of the cerebellum to injury stemming from a thiamine deficient diet or impaired thiamine utilization.
Similar content being viewed by others
References
Singleton CK, Martin PR. Molecular mechanisms of thiamine utilization. Curr Mol Med. 2001;1:197–207.
Martin PR, Singleton CK, Hiller-Sturmhofel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27:134–42.
Thomson AD. Mechanisms of vitamin deficiency in chronic alcohol misusers and the development of the Wernicke-Korsakoff syndrome. Alcohol Alcohol Suppl. 2000;35:2–7.
Butterworth RF. Effects of thiamine deficiency on brain metabolism: Implications for the pathogenesis of the Wernicke-Korsakoff syndrome. Alcohol Alcohol. 1989;24:271–9.
Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome and related neurologic disorders due to alcoholism and malnutrition. 2nd ed. Philadelphia, PA: FA Davis, 1989.
Harper CG, Butterworth RF. Nutritional and metabolic disorders. In: Graham DI, Lantos PL, editors. Greenfield’s Neuropathology. London: Arnold; 1997. pp 601–55.
Todd K, Butterworth RF. Mechanisms of selective neuronal cell death due to thiamine deficiency. Ann N Y Acad Sci. 1999;893:404–11.
Courchesne E, Townsend J, Akshoomoff NA, et al. Impairment in shifting attention in autistic and cerebellar patients. Behav Neurosci. 1994;108:848–65.
Schmahmann JD. Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–78.
Nolte. The Human Brain. 3rd ed. St Louis: Mosby Year Book, 1999.
Andersen BB, Korbo L, Pakkenberg B. A quantitative study of the human cerebellum with unbiased stereological techniques. J Comp Neurol. 1992;326:549–60.
Mayhew TM. Accurate prediction of Purkinje cell number from cerebellar weight can be achieved with the fractionator. J Comp Neurol. 1991;308:162–8.
Adeyinka AO, Akinyinka OO, Falade AG. Computerized tomography measures of brain slice area and ventricular sizes in protein energy malnutrition: A preliminary study. West Afr J Med. 1996;15:232–6.
Kohn MR, Ashtari M, Golden NH, et al. Structural brain changes and malnutrition in anorexia nervosa. Ann N Y Acad Sci. 1997;28:398–9.
Reuler JB, Girard DE, Cooney TG. Current concepts. Wernicke’s encephalopathy. N Engl J Med. 1985;312:1035–9.
Ogershok PR, Rahman A, Nestor S, Brick J. Wernicke encephalopathy in nonalcoholic patients. Am J Med Sci. 2002;323:107–11.
Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: Relation to ataxia. Neuropsychology. 2000;14:341–52.
Alcaide ML, Jayaweera D, Espinoza L, Kolber M. Wernicke’s encephalopathy in AIDS: A preventable cause of fatal neurological deficit. Int J STD AIDS. 2003;14:712–13.
Chung TI, Kim JS, Park SK, Kim BS, Ahn KJ, Yang DW. Diffusion weighted MR imaging of acute Wernicke’s encephalopathy. Eur J Radiol. 2003;45:256–8.
Macleod AD. Wernicke’s encephalopathy and terminal cancer: Case report. Palliat Med. 2000;14:217–18.
Nicolas JM, Fernandez-Sola J, Robert J, et al. High ethanol intake and malnutrition in alcoholic cerebellar shrinkage. QJM. 2000;93:449–56.
San Sebastian M, Jativa R. Beriberi in a well-nourished Amazonian population. Acta Trop. 1998;70:193–6.
Sharma S, Sumich PM, Francis IC, Kiernan MC, Spira PJ. Wernicke’s encephalopathy presenting with upbeating nystagmus. J Clin Neurosci. 2002;9:476–8.
Eliakim R, Abulafia O, Sherer DM. Hyperemesis gravidarum: A current review. Am J Perinatol. 2000;17:207–18.
Luke B, Johnson T, Petrie R. The prenatal diet. In: Pioli S, Cardone K, Dabrowski L, editors. Clinical maternal-fed nutrition. 1st ed. Boston: Little, Brown, and Company; 1993. pp 123–46.
Togay-Isikay C, Yigit A, Mutluer N. Wernicke’s encephalopathy due to hyperemesis gravidarum: An under-recognised condition. Aust N Z J Obstet Gynaecol. 2001;41:453–6.
Onishi H, Kawanishi C, Onose M, et al. Successful treatment of Wernicke encephalopathy in terminally ill cancer patients: report of 3 cases and review of the literature. Support Care Cancer. 2004;12:604–08.
Murata T, Fujito T, Kimura H, Omori M, Itoh H, Wada Y. Serial MRI and (1)H-MRS of Wernicke’s encephalopathy: report of a case with remarkable cerebellar lesions on MRI. Psychiatry Res. 2001;108:49–55.
Weidauer S, Rosler A, Zanella FE, Lanfermann H. Diffusionweighted imaging in Wernicke encephalopathy associated with stomach cancer: Case report and review of the literature. Eur Neurol. 2004;51:55–7.
Butterworth RF, Gaudreau C, Vincelette J, Bourgault AM, Lamothe F, Nutini AM. Thiamine deficiency and Wernicke’s encephalopathy in AIDS. Metab Brain Dis. 1991;6:207–12.
Nishimune T, Watanabe Y, Okazaki H, Akai H. Thiamin is decomposed due to Anaphe spp. entomophagy in seasonal ataxia patients in Nigeria. J Nutr. 2000;130:1625–8.
Adamolekun B, Adamolekun WE, Sonibare AD, Sofowora G. A double-blind, placebo controlled study of the efficacy of thiamine hydrochloride in a seasonal ataxia in Nigerians. Neurology. 1994;44:549–51.
Lee H, Tarter J, Holburn GE, Price RR, Weinstein DD, Martin PR. In vivo localized proton NMR spectroscopy of thiamine-deficient rat brain. Magn Reson Med. 1995;34:313–18.
Ambrose ML, Bowden SC, Whelan G. Thiamin treatment and working memory function of alcohol-dependent people: Preliminary findings. Alcohol Clin Exp Res. 2001;25:112–16.
Parkin AJ, Blunden J, Rees JE, Hunkin NM. Wernicke-Korsakoff syndrome of nonalcoholic origin. Brain Cogn. 1991;15:69–82.
Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: A retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49:341–5.
Agabio R. Thiamine administration in alcohol-dependent patients. Alcohol Alcohol. 2005;40:155–6.
Thomson AD, Cook CC, Touquet R, Henry JA. The Royal College of Physicians report on alcohol: Guidelines for managing Wernicke’s encephalopathy in the accident and Emergency Department. Alcohol Alcohol. 2002;37:513–21.
Feinberg JF. The Wernicke-Korsakoff syndrome. Am Fam Physician. 1980;22:129–33.
Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome. A clinical and pathological study of 245 patients, 82 with post-mortem examinations. Contemp Neurol Ser. 1971;7:1–206.
Ishida T, Murayama S, Inoue K, Machinami R, Tomonaga M, Mannen T. Degeneration of posterior column nucleus, inferior olivary nucleus and cerebellar cortex: System degeneration of paraneoplastic disease? Clin Neuropathol. 1990;9:262–7.
Smith TW, DeGirolami U, Henin D, Bolgert F, Hauw JJ. Human immunodeficiency virus (HIV) leukoencephalopathy and the microcirculation. J Neuropathol Exp Neurol. 1990;49:357–70.
Baker KG, Harding AJ, Halliday GM, Kril JJ, Harper CG. Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke’s encephalopathy. Neuroscience. 1999;91:429–38.
Butterworth RF. Pathophysiology of cerebellar dysfunction in the Wernicke-Korsakoff syndrome. Can J Neurol Sci. 1993;20:S123–6.
Rugilo CA, Uribe Roca MC, Zurru MC, Capizzano AA, Pontello GA, Gatto EM. Proton MR spectroscopy in Wernicke encephalopathy. AJNR Am J Neuroradiol. 2003;24:952–5.
Andersen BB. Reduction of Purkinje cell volume in cerebellum of alcoholics. Brain Res. 2004;1007:10–18.
Phillips SC, Harper C, Kril J. A quantitative histological study of the cerebellar vermis in alcoholic patients. Brain. 1987;110:301–14.
Budka H. Neuropathology of human immunodeficiency virus infection. Brain Pathol. 1991;1:163–75.
Schwenk J, Gosztonyi G, Thierauf P, Iglesias J, Langer E. Wernicke’s encephalopathy in two patients with acquired immunodeficiency syndrome. J Neurol. 1990;237:445–7.
Rosemberg S, Lopes MB, Tsanaclis AM. Neuropathology of acquired immunodeficiency syndrome (AIDS). Analysis of 22 Brazilian cases. J Neurol Sci. 1986;76:187–98.
Mukherjee AB, Ghazanfari A, Svoronos S, Staton RC, Nakada T, Kwee IL. Transketolase abnormality in tolazamideinduced Wernicke’s encephalopathy. Neurology. 1986;36:1508–10.
Casirola D, Ferrari G, Gastaldi G, Patrini C, Rindi G. Transport of thiamine by brush-border membrane vesicles from rat small intestine. J Physiol. 1988;398:329–39.
Iwashima A, Wakabayashi Y, Nose Y. Thiamine transport mutants ofSaccharomyces cerevisiae. Biochim Biophys Acta. 1975;413:243–7.
Yoshioka K. Some properties of the thiamine uptake system in rat hepatocytes. Biochim Biophys Acta. 1984;778:201–09.
Irle E, Markowitsch HJ. Widespread neuroanatomical damage and learning deficits following chronic alcohol consumption or vitamin-B1 (thiamine) deficiency in rats. Behav Brain Res. 1983;9:277–94.
Mulholland PJ, Self RL, Stepanyan TD, Little HJ, Littleton JM, Prendergast MA. Thiamine deficiency in the pathogenesis of chronic ethanol-associated cerebellar damage in vitro. Neuroscience. 2005;135:1129–39.
Pannunzio P, Hazell AS, Pannunzio M, Rao KV, Butterworth RF. Thiamine deficiency results in metabolic acidosis and energy failure in cerebellar granule cells: an in vitro model for the study of cell death mechanisms in Wernicke’s encephalopathy. J Neurosci Res. 2000;62:286–92.
Witt ED. Neuroanatomical consequences of thiamine deficiency: A comparative analysis. Alcohol Alcohol. 1985;20:201–21.
Hazell AS, Todd KG, Butterworth RF. Mechanisms of neuronal cell death in Wernicke’s encephalopathy. Metab Brain Dis. 1998;13:97–122.
Yokote K, Miyagi K, Kuzuhara S, Yamanouchi H, Yamada H. Wernicke encephalopathy: follow-up study by CT and MR. J Comput Assist Tomogr. 1991;15:835–8.
Schroth G, Wichmann W, Valavanis A. Blood-brain-barrier disruption in acute Wernicke encephalopathy: MR findings. J Comput Assist Tomogr. 1991;15:1059–61.
Hakim AM, Pappius HM. Sequence of metabolic, clinical, and histological events in experimental thiamine deficiency. Ann Neurol. 1983;13:365–75.
Hakim AM. The induction and reversibility of cerebral acidosis in thiamine deficiency. Ann Neurol. 1984;16:673–9.
Langlais PJ, Mair RG. Protective effects of the glutamate antagonist MK-801 on pyrithiamine-induced lesions and amino acid changes in rat brain. J Neurosci. 1990;10:1664–74.
Todd KG, Butterworth RF. Evaluation of the role of NMDA-mediated excitotoxicity in the selective neuronal loss in experimental Wernicke encephalopathy. Exp Neurol. 1998;149:130–8.
Bettendorff L, Sluse F, Goessens G, Wins P, Grisar T. Thiamine deficiency-induced partial necrosis and mitochondrial uncoupling in neuroblastoma cells are rapidly reversed by addition of thiamine. J Neurochem. 1995;65:2178–84.
Desjardins P, Butterworth RF. Role of mitochondrial dysfunction and oxidative stress in the pathogenesis of selective neuronal loss in Wernicke’s encephalopathy. Mol Neurobiol. 2005;31:17–25.
Langlais PJ, Anderson G, Guo SX, Bondy SC. Increased cerebral free radical production during thiamine deficiency. Metab Brain Dis. 1997;12:137–43.
Wang JJ, Hua Z, Fentress HM, Singleton CK. JNK1 is inactivated during thiamine deficiency-induced apoptosis in human neuroblastoma cells. J Nutr Biochem. 2000;11:208–15.
Kril JJ. Neuropathology of thiamine deficiency disorders. Metab Brain Dis. 1996;11:9–17.
Tretter L, Adam-Vizi V. Alpha-ketoglutarate dehydrogenase: A target and generator of oxidative stress. Philos Trans R Soc Lond B Biol Sci. 2005;360:2335–45.
Vogel S, Hakim AM. Effect of nimodipine on the regional cerebral acidosis accompanying thiamine deficiency in the rat. J Neurochem. 1988;51:1102–10.
Blass JP, Gibson GE. Abnormality of a thiamine-requiring enzyme in patients with Wernicke-Korsakoff syndrome. N Engl J Med. 1977;297:1367–70.
Martin PR, McCool BA, Singleton CK. Genetic sensitivity to thiamine deficiency and development of alcoholic organic brain disease. Alcohol Clin Exp Res. 1993;17:31–37.
Mukherjee AB, Svoronos S, Ghazanfari A, et al. Transketolase abnormality in cultured fibroblasts from familial chronic alcoholic men and their male offspring. J Clin Invest. 1987;79:1039–43.
Spruill SC, Kuller JA. Hyperemesis gravidarum complicated by Wernickes encephalopathy. Obstet Gynecol. 2002;99:875–7.
Husami T, Abumrad NN. Adverse metabolic consequences of nutritional support: Micronutrients. Surg Clin North Am. 1986;66:1049–69.
Flink EB. Magnesium deficiency in alcoholism. Alcohol Clin Exp Res. 1986;10:590–4.
Abel EL. Maternal alcohol consumption and spontaneous abortion. Alcohol Alcohol. 1997;32:211–19.
Windham GC, Von Behren J, Fenster L, Schaefer C, Swan SH. Moderate maternal alcohol consumption and risk of spontaneous abortion. Epidemiology. 1997;8:509–14.
Adinoff B, Ruether K, Krebaum S, Iranmanesh A, Williams MJ. Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcohol Clin Exp Res. 2003;27:1420–7.
Stein-Behrens BA, Lin WJ, Sapolsky RM. Physiological elevations of glucocorticoids potentiate glutamate accumulation in the hippocampus. J Neurochem. 1994;63:596–602.
Horner HC, Packan DR, Sapolsky RM. Glucocorticoids inhibit glucose transport in cultured hippocampal neurons and glia. Neuroendocrinology. 1990;52:57–64.
Yusim A, Ajilore O, Bliss T, Sapolsky R. Glucocorticoids exacerbate insult-induced declines in metabolism in selectively vulnerable hippocampal cell fields. Brain Res. 2000;870:109–17.
Zimitat C, Nixon PF. Glucose loading precipitates acute encephalopathy in thiamine-deficient rats. Metab Brain Dis. 1999;14:1–20.
Hazell AS, Butterworth RF, Hakim AM. Cerebral vulnerability is associated with selective increase in extracellular glutamate concentration in experimental thiamine deficiency. J Neurochem. 1993;61:1155–8.
Hazell AS, Rao KV, Danbolt NC, Pow DV, Butterworth RF. Selective down-regulation of the astrocyte glutamate transporters GLT-1 and GLAST within the medial thalamus in experimental Wernicke’s encephalopathy. J Neurochem. 2001;78:560–8.
Maschke M, Weber J, Bonnet U, et al. Vermal atrophy of alcoholics correlate with serum thiamine levels but not with dentate iron concentrations as estimated by MRI. J Neurol. 2005;252:704–11.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mulholland, P.J. Susceptibility of the cerebellum to thiamine deficiency. Cerebellum 5, 55–63 (2006). https://doi.org/10.1080/14734220600551707
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1080/14734220600551707