Abstract
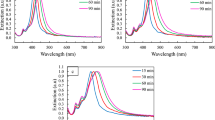
Colloidal silver nanocubes (NCs) were successfully synthesized by reduction of silver nitrate with ethylene glycol and polyvinylpyrrolidon as capping agent. The effect of solar light irradiation on the formation and morphology of silver NCs was investigated. Moreover, altering the amount of sodium sulfide was used to control the morphology and shape of primary silver seeds. Scanning electron microscopy, transmitting electron microscopy, X-ray diffraction and UV-vis spectroscopy were used to characterize silver NCs. The samples prepared under the solar light irradiation do not possess cubic shape while highly monodispersed silver NCs were obtained in dark room conditions. For dark room synthesis, a decrease of the amount of Na2S by only 10 μL resulted in formation of mixture of silver nanospheres and nanowires in addition to NCs instead of the monodispersed silver NCs. However, similar increase of the amount of sodium sulfide results in distortion of cubic geometry of particles. The results suggest that solar light has a negative effect on the shape evolution of the primary silver seeds.
Similar content being viewed by others
References
Khlebtsov, B.N. and Khlebtsov, N.G., Colloid J., 2011, vol. 73, p. 118.
Eustis, S. and El-Sayed, M.A., Chem. Soc. Rev., 2006, vol. 35, p. 209.
Yeh, Y.-C., Creran, B., and Rotello, V.M., Nanoscale, 2012, vol. 4, p. 1871.
Long, N.V., Chien, N.D., Hirata, H., et al., J. Cryst. Growth, 2011, vol. 320, p. 78.
Pradeep, T., Thin Solid Films, 2009, vol. 517, p. 6441.
Cuenya, B.R., Thin Solid Films, 2010, vol. 518, p. 3127.
Ashkarran, A.A., Ghavami, M., Aghaverdi, H., et al., Chem. Res. Toxicol., 2012, vol. 25, p. 1231.
Sun, Y. and Xia, Y., Science (Washington, D. C.), 2002, vol. 298, p. 2176.
Olenin, A.Y., Krutyakov, Yu.A., Kudrinskii, A.A., and Lisichkin, G.V., Colloid J., 2008, vol. 70, p. 71.
Skrabalak, S.E., Au, L., Li, X., and Xia, Y., Nat. Protoc., 2007, vol. 2, p. 2182.
Skrabalak, S.E., Chen, J., Sun, Y., et al., Acc. Chem. Res., 2008, vol. 41, p. 1587.
Xia, Y., Xiong, Y., Lim, B., and Skrabalak, S.E., Angew. Chem., Int. Ed. Engl., 2009, vol. 48, p. 60.
Karpov, S., Gerasimov, V.S., Grachev, A.S., et al., Colloid J., 2007, vol. 69, p. 170.
Cobley, C.M., Skrabalak, S.E., and Campbell, D.J., Plasmonics, 2009, vol. 4, p. 171.
Siekkinen, A.R., McLellan, J.M., Chen., J., and Xia, Y., Chem. Phys. Lett., 2006, vol. 432, p. 491.
Wiley, B., Sun, Y., Mayers, B., and Xia, Y., Chem. Eur. J., 2005, vol. 11, p. 454.
Wiley, B.J., Im, S.H., Li, Z.Y., et al., J. Phys. Chem. B, 2006, vol. 110, p. 15666.
Xia, Y., Nat. Mater., 2008, vol. 7, p. 758.
Panfilova, E.V., Khlebtsov, B.N., Burov, A.M., et al., Colloid J., 2012, vol. 74, p. 99.
Chen, C., Wang, L., Yu, H., et al., Nanotechnology, 2007, vol. 18, p. 115612.
Chen, D., Qiao, X., Qiu, X., et al., Nanotechnology, 2010, vol. 21, p. 025607.
Heinzel, T., Mesoscopic Electronics in Solid State Nanostructures, Weinheim Wiley-VCH, 2003.
Ashkarran, A.A., Curr. Appl Phys., 2010, vol. 10, p. 1442.
Thaxton, C.S. and Mirkin, C.A., Nat. Biotechnol., 2005, vol. 23, p. 681.
McMahon, J.M., Wang, Y., Sherry, L.J., et al., J. Phys. Chem. C, 2009, vol. 113, p. 2731.
Xie, Y., Heo, S.H., Kim, Y.N., et al., Nanotechnology, 2010, vol. 21, p. 015703.
Rycenga, M., McLellan, J.M., and Xia, Y., Adv. Mater. (Weinheim, Fed. Repub. Ger.), 2008, vol. 20, p. 2416.
Cortie, M.B., Liu, F., Arnold, M.D., et al., Langmuir, 2012, vol. 28, p. 9103.
Sherry, L.J., Chang, S.H., Schatz, G.C., et al., Nano Lett., 2005, vol. 5, p. 2034.
Rycenga, M., Xia, X., Moran, C.H., et al., Angew. Chem., Int. Ed. Engl., 2011, vol. 50, p. 5473.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Ashkarran, A.A., Daemi, S. & Derakhshi, M. Destructive effect of solar light on morphology of colloidal silver nanocubes. Colloid J 78, 577–585 (2016). https://doi.org/10.1134/S1061933X16050021
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X16050021