Abstract
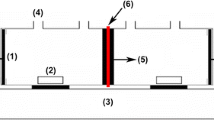
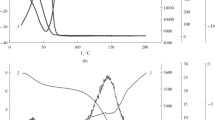
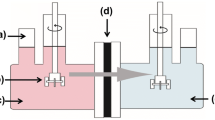
The electrokinetic properties and selectivity of an acetyl cellulose membrane with respect to 0.0001 mol/L sodium chloride solutions in water–ethanol mixtures have been studied. The electrical conductivity, streaming potential, and filtration and selectivity characteristics of the membrane have been measured. It has been found that, in solutions with alcohol contents of 4 and 12%, the membrane selectivity with respect to sodium chloride is increased and decreased relative to that in an aqueous solution, respectively. No correlation between the membrane selectivity and its surface charge has been observed. The membrane has been found to possess a slight selectivity (20–26%) with respect to ethanol. It has been hypothesized that the solvation enthalpy of electrolyte ions changes differently in a free solution and membrane pores at different contents of ethanol in the mixtures, thereby affecting the membrane selectivity.



Similar content being viewed by others
REFERENCES
Martynov, G.A., Starov, V.M., and Churaev, N.V., Kolloidn. Zh., 1980, vol. 42, p. 489.
Derjaguin, B.V., Churaev, N.D., and Martynov, G.A., J. Colloid Interface Sci., 1980, vol. 75, p. 419.
Churaev, N.V. and Derjaguin, B.V., Zh. Vses. Khim. O-va.im.D.I. Mendeleeva, 1987, vol. 32, p. 614.
Yaroshchuk, A.E., Adv. Colloid Interface. Sci., 2000, vol. 85, p. 193.
Spiegler, K.S., Trans. Faraday Soc., 1958, vol. 54, p. 1408.
Merten, U., Desalination by Reverse Osmosis, Cambridge: MIT, 1966.
Manning, G.S., J. Phys. Chem., 1972, vol. 76, p. 393.
Donnan, F.G., J. Membr. Sci., 1995, vol. 100, p. 45.
Schmid, G. and Schwars, H., Z. Elektrochem., 1952, vol. 52, p. 35.
Hoffer, E. and Kedem, O., J. Phys. Chem., 1972, vol. 76, p. 3638.
Bowen, W.R. and Mukhtar, H., J. Membr. Sci., 1996, vol. 112, p. 263.
Bowen, W.R. and Welfoot, J.S., Chem. Eng. Sci., 2002, vol. 57, p. 1121.
Vezzani, D. and Bandini, S., Desalination, 2002, vol. 149, p. 477.
Bandini, S. and Vezzani, D., Chem. Eng. Sci., 2003, vol. 58, p. 3303.
Bandini, S., J. Membr. Sci., 2005, vol. 264, p. 75.
Bruni, L. and Bandini, S., J. Membr. Sci., 2008, vol. 308, p. 136.
Szymczyk, A. and Fievet, P., J. Membr. Sci., 2005, vol. 252, p. 77.
Born, M., Z. Phys., 1920, vol. 1, p. 45.
Stokes, R.H., J. Am. Chem. Soc., 1964, vol. 64, p. 979.
Kornyshev, A.A., Tsitsuashvili, G.I., and Yaroshchuk, A.E., Elektrokhimiya, 1989, vol. 25, p. 1037.
Bernal, J.D. and Fowler, R.H., J. Chem. Phys., 1933, vol. 1, p. 515.
Krestov, G.A., Ionnaya sol’vatatsiya (Ion Solvation), Moscow: Nauka, 1987.
Skanavi, G.I., Fizika dielektrikov (Physics of Dielectrics), Moscow: Gos. Izd. Tekh.-Teor. Lit., 1949.
Ontaki, H., Yamaguchi, T., and Maeda, M., Bull. Chem. Soc. Jpn., 1976, vol. 49, p. 701.
Krishtalik, L.I., Alpatova, N.M., and Ovsyannikova, E.V., Elektrokhimiya, 1990, vol. 26, p. 429.
Krishtalik, L.I., Alpatova, N.M., and Ovsyannikova, E.V., Elektrokhimiya, 1990, vol. 26, p. 436.
Mishustin, A.I., Mozalevskaya, V.A., and Ponomarev, V.P., Zh. Fiz. Khim., 1989, vol. 63, p. 1345.
Manin, N.G. and Korolev, V.P., Russ. J. Phys. Chem., 2002, vol. 76, p. 177.
Samoilov, O.Ya., Struktura vodnykh rastvorov elektrolitov i gidratatsiya ionov (The Structure of Electrolyte Aqueous Solutions and Ion Hydration), Moscow: Akad. Nauk SSSR, 1957.
Ding, M., Szymczyk, A., and Ghouf, A., Desalination, 2015, vol. 368, p. 76.
Etzler, F.M., J. Colloid Interface Sci., 1983, vol. 92, p. 43.
Ovchinnikov, A.L., Timashev, S.F., and Belyi, F.L., Kinetika diffuzionno-kontroliruemykh protsessov (Kinetics of Diffusion Controlled Processes), Moscow: Khimiya, 1986.
Atamanenko, I.D. and Bryk, Yu.I., Kolloidn. Zh., 1991, vol. 53, p. 336.
Sun, Q. and Zheng, H.-F., Chin. Phys. Lett., 2006, vol. 23, p. 3022.
Chen, I.T., Sessoms, D.A., Sherman, Z., Choi, E., Vincent, O., and Stroock, A.D., J. Phys. Chem. B, 2016, vol. 120, p. 5209.
Ivanova, N.I., Vakar, N.T., and Pertsov, N.V., Kolloidn. Zh., 1987, vol. 49, p. 1348.
Chankina, T.I. and Parfenyuk, V.I., Russ. J. Phys. Chem., 2011, vol. 85, p. 1307.
Chankina, T.I. and Parfenyuk, V.I., Russ. J. Electrochem., 2008, vol. 44, p. 1162.
Parfenyuk, V.I., Russ. J. Phys. Chem., 2005, vol. 79, p. 898.
Kessler, Yu.M. and Zaitsev, A.L., Sol’vofobnye effekty. Teoriya, eksperiment, praktika (Solvophobic Effects. Theory, Experiment, Practice), Leningrad: Khimiya, 1989.
Zhukov, A.N. and Fedorova, I.L., Colloid J., 2004, vol. 66, p. 292.
Kosmulski, M. and Matijevic, E., Langmuir, 1992, vol. 8, p. 1060.
Pihlajamaki, A., Laakso, T., and Manttari, M., Proc. Eng., 2012, vol. 44, p. 1502.
Zhukov, A.N. and Fedorova, I.L., Kolloidn. Zh., 1990, vol. 52, p. 781.
Sabbatovskii, K.G., Sobolev, V.D., and Churaev, N.V., Kolloidn. Zh., 1991, vol. 53, p. 74.
Sabbatovskii, K.G., Sobolev, V.D., and Churaev, N.V., Kolloidn. Zh., 1991, vol. 53, p. 403.
Rodionova, I.A., Shkol’nikov, E.I., and Volkov, V.V., Colloid J., 2005, vol. 67, p. 469.
Funding
This work was performed within the framework of a state order to the Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by A. Kirilin
Rights and permissions
About this article
Cite this article
Sabbatovskii, K.G., Sergeeva, I.P. & Sobolev, V.D. The Effect of Aqueous–Ethanol Sodium Chloride Solutions on the Selectivity and Electrosurface Properties of an Acetyl Cellulose Membrane. Colloid J 81, 747–753 (2019). https://doi.org/10.1134/S1061933X19060164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1061933X19060164