Abstract—
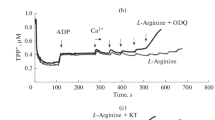
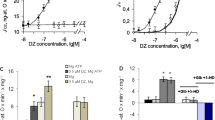
The involvement of the mitochondrial calcium-dependent NO synthase (mtNOS) in the regulation of mitochondrial respiration has not been sufficiently studied. Moreover, the possible functioning of a mitochondrial signaling system involving mtNOS/guanylate cyclase (GC)/protein kinase G (PKG) and the impact of this system (mtNOS/GC/PKG-SS) on mitochondrial respiration have not yet been analyzed. To investigate this issue, we performed experiments on isolated rat liver using specific inhibitors of NOS, GC, and PKG. The high rate of mitochondrial respiration was supported by pyruvate and glutamate or by succinate in the presence of hexokinase, glucose, and ADP. It was shown that L-arginine and the NO donor sodium nitroprusside (SNP) exert concentration-dependent effects on the mitochondrial respiration rate. At low concentrations, L‑arginine (up to 200 μM) and SNP (up to100 μM) activated mitochondrial respiration. The inhibitors of NOS, GC, and PKG eliminated this effect indicating that mtNOS/GC/PKG-SS is involved in the activation of respiration. At high concentrations, L-arginine and SNP, on the contrary, inhibited respiration. Under these conditions, the inhibitors of GC and PKG enhanced the inhibition of respiration, which indicates an opposite effect of the excess of NO and PKG on the mitochondrial respiration. The results suggest that the functioning of calcium-dependent mtNOS/GC/PKG-SS can ensure the activation of respiration at low concentrations of L-arginine or SNP in the medium.




Similar content being viewed by others
REFERENCES
Cleeter M.W., Cooper J.M., Darley-Usmar V.M.; Moncada S., Schapira A.H. 1994. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett.345 (1), 50–54. https://doi.org/10.1016/0014-5793(94)00424-2
Giulivi C. 1998. Functional implications of nitric oxide produced by mitochondria in mitochondrial metabolism. Biochem. J.332, 673–679.
Brown G.C. 2001. Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase. Biochim. Biophys.Acta. 1504 (1), 46–57.https://doi.org/10.1016/S0005-2728(00)00238-3
Parihara M.S., Nazarewicza R.R., Kincaida E., Bringoldb U., Ghafourifara P. 2008. Association of mitochondrial nitric oxide synthase activity with respiratory chain complex I. Biochem. Biophys. Res. Commun.366 (1), 23–28. https://doi.org/10.1016/j.bbrc.2007.11.056
Giulivi C., Kato K., Cooper C.E. 2006. Nitric oxide regulation of mitochondrial oxygen consumption I: Cellular physiology. Am. J. Physiol. Cell. Physiol. 291 (6), C1225–C1231. https://doi.org/10.1152/ajpcell.00307.2006
Palacios-Callender M., Hollis V., Frakich N., Mateo J., Moncada S. 2007. Cytochrome c oxidase maintains mitochondrial respiration during partial inhibition by nitric oxide. J. Cell Sci.120 (Pt 1), 160–165. https://doi.org/10.1242/jcs.03308
Kanai A., Epperly M., Pearce L., Birder L., Zeidel M., Meyers S., Greenberger J., de Groat W., Apodaca G., Peterson J. 2004. Differing roles of mitochondrial nitric oxide synthase in cardiomyocytes and urothelial cells. Am. J. Physiol. Heart Circ. Physiol.286 (1), H13–H21. Erratum in: Am. J. Physiol. Heart Circ. Physiol.296 (2), H536.
Elfering S.L., Sarkela T.M., Giulivi C. 2004. Biochemistry of mitochondrial nitric-oxide synthase. J. Biol. Chem.277, 38079–38086.
Kanai A.J., Pearce L.L, Clemens P.R., Birder L.A., VanBibber M.M., Choi S.Y., de Groat W.C., Peterson J. 2004. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc. Natl. Acad. Sci. USA.98, 14126–14131.
Boveris A., Valdez L.B., Zaobornyj T., Bustamante J. 2006. Mitochondrial metabolic states regulate nitric oxide and hydrogen peroxide diffusion to the cytosol. Biochim. Biophys. Acta.1757 (5–6), 535–542.https://doi.org/10.1016/j.bbabio.2006.02.010
Giulivi C. 2007. Mitochondria as generators and targets of nitric oxide. Novartis Fdn. Symp.287, 92–100.
Traaseth N., Elfering S, Solien J., Haynes V., Giulivi C. 2004. Role of calcium signaling in the activation of mitochondrial nitric oxide synthase and citric acid cycle. Biochim. Biophys. Acta. 1658 (1–2), 64–71. https://doi.org/10.1016/j.bbabio.2004.04.015
Lemasters J.J., Tom P., Theruvath I., Zhong Z., Nieminen A.-L. 2009. Mitochondrial calcium and the permeability transition in cell death, Biochim. Biophys. Acta.1787, 1395–1401. https://doi.org/10.1016/j.bbabio.2009.06.009
Halestrap A.P. 2010. A pore way to die: The role of mitochondria in reperfusion injury and cardioprotection. Biochem. Soc. Trans. 38 (4), 841–860.https://doi.org/10.1042/BST0380841
Sardanelli A.M., Technikova-Dobrova Z., Scacco S.C., Speranza F., Papa S. 1995. Characterization of proteins phosphorylated by the cAMP-dependent protein kinase of bovine heart mitochondria. FEBS Lett. 377 (3), 470–474. https://doi.org/10.1016/0014-5793(95)01407-1
Di Benedetto G., Pendin D., Greotti E., Pizzo P., Pozzan T. 2014. Ca2+ and cAMP cross-talk in mitochondria. J. Physiol.592 (2), 305–312. https://doi.org/10.1113/jphysiol.2013.259135
Ould Amer Y., Hebert-Chatelain E. 2018. Mitochondrial cAMP-PKA signaling: What do we really know? Biochim. Biophys. Acta. 1859 (9), 868–877. https://doi.org/10.1016/j.bbabio.2018.04.005
Rodríguez-Juárez F., Aguirre E., Cadenas S. 2007. Relative sensitivity of soluble guanylate cyclase and mitochondrial respiration to endogenous nitric oxide at physiological oxygen concentration. Biochem. J.405, 223–231.https://doi.org/10.1042/BJ20070033
Lim S., Smith K.R., Lim S.‑T. S., Tian R., Lu J., Tan M. 2016. Regulation of mitochondrial functions by protein phosphorylation and dephosphorylation. Cell Biosci.6 (25). eCollection 2016. https://doi.org/10.1186/s13578-016-0089-3
Su C.C., Yang J.Y., Leu H.B., Chen, Y., Wang P.H. 2012. Mitochondrial Akt-regulated mitochondrial apoptosis signaling in cardiac muscle cells. Am. J. Physiol. Heart. Circ. Physiol.302 (3), H716–H723. https://doi.org/10.1152/ajpheart.00455.2011
ACKNOWLEDGMENTS
The work was supported by the Russian Foundation for Basic Research (projects nos. 14-04-01695, VD, and 14-04-01597m, EG). The authors thank M.A. Simonova, M.H. Gali-mova, and A.I. Sergeeva for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
All procedures were performed in accordance with the European Communities Council Directive (November 24, 1986; 86/609/EEC) and the Declaration on humane treatment of animals. The experimental protocol was approved by the Bioethics Committee of the Institute of Theoretical and Experimental Biophysics, RAS.
Additional information
Translated by E. Puchkov
Abbreviations: MPTP, mitochondrial permeability transition pore; GC, guanylate cyclase; mtNOS, mitochondrial NO-synthase; PKG1, protein kinase G; COX, cytochrome-c-oxidase; SNP, sodium nitroprusside; cGMP, cyclic guanosine monophosphate; ODQ, selective inhibitor of soluble guanylate cyclase (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one); 7-NI, an inhibitor of endothelial and neuronal NO-synthases (7-nitroindasole; N‑[[3-(Aminomethyl)phenyl]methyl]-ethanimidamide dihydrochloride); 1400W, selective inhibitor of inducible NO-synthase; KT, selective protein kinase G inhibitor KT5823.
Rights and permissions
About this article
Cite this article
Dynnik, V.V., Grishina, E.V. & Fedotcheva, N.I. Implication of Mitochondrial NO/cGMP/PKG Signaling System in the Activation and Inhibition of Mitochondrial Respiration by L-Arginine and NO Donors. Biochem. Moscow Suppl. Ser. A 13, 334–340 (2019). https://doi.org/10.1134/S1990747819040056
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747819040056