Abstract
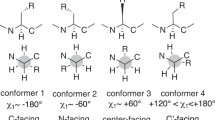
The geometric parameters and relative stability of γ- and β-loop conformers of oligomers based on alanine were determined by the Kohn-Sham method (the B3LYP/6–31+G** approximation). The three-dimensional architecture of the β-folded structure and protein α-helix was reproduced using Kohn-Sham calculations with periodic boundary conditions. The Bader quantum-topological molecular structure theory was used to reveal and quantitatively characterize noncovalent interatomic interactions in the secondary structures of model peptides under consideration. Earlier unnoticed additional noncovalent interactions stabilizing the structures under consideration were revealed. In β-loops, these are C-H⋯O and H⋯H interactions, and, in antiparallel β-folded structures, these are weak Cβ-H⋯H-Cβ interactions between side chains. Additional weak bonding interaction between C=O groups in position i and H-Cβ groups in position i + 3 was revealed for the protein α-helix; this interaction is usually ignored in amino acid folding simulations with the use of classic force fields.
Similar content being viewed by others
References
J. Bella and H. M. Berman, J. Mol. Biol. 264, 734 (1996).
A. K. Thakur and R. Kishore, Biopolymers 81, 440 (2006).
E. Vass, M. Hollósi, F. Besson, et al., Chem. Rev. 103, 1917 (2003).
V. Brenner, F. Piuzzi, I. Dimicoli, et al., Angev. Chem. Int. Ed. 47, 2463 (2007).
V. Brenner, F. Piuzzi, I. Dimicoli, et al., J. Phys. Chem. A 111, 7347 (2007).
I. Compagnon, J. Oomens, G. Meijer, et al., J. Am. Chem. Soc. 128, 3592 (2006).
A. Perczel, M. A. McAllister, P. Csaszar, et al., J. Am. Chem. Soc. 115, 4849 (1993).
R. Kaschner and D. Hohl, J. Phys. Chem. A 102, 5111 (1998).
I. Topol, S. K. Burt, T. Deretey, et al., J. Am. Chem. Soc. 123, 6054 (2001).
R. Vargas, J. Garza, B. P. Hay, et al., J. Phys. Chem. A 106, 3214 (2002).
R. Improta and V. Barone, J. Comput. Chem. 25, 1333 (2004).
Z. Varga and A. Kovács, Int. J. Quantum Chem. 105, 302 (2005).
S. Scheiner, J. Phys. Chem. B 110, 18670 (2006).
R. Improta, V. Barone, K. N. Kudin, et al., J. Am. Chem. Soc. 123, 3311 (2001).
J. Ireta, J. Neugebauer, M. Scheffler, et al., J. Phys. Chem. B 107, 1432 (2003).
J. Rossmeisl, I. Kristensen, M. Gregensen, et al., J. Am. Chem. Soc. 125, 16383 (2003).
T.-H. Tang, E. Deretey, S. J. K. Jensen, et al., Eur. Phys. J. D 37, 217 (2006).
R. Parthasarathi, S. S. Raman, V. Subramanian, et al., J. Phys. Chem. A 111, 7141 (2007).
R. F. W. Bader, Atoms in Molecules. A Quantum Theory (Oxford Univ., New York, 1990).
A. A. Granovsky, PC GAMESS version 7.0, http://classic.chem.msu.su/gran/gamess/index.html.
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, et al., J. Comput. Chem. 14, 1347 (1993).
J. Rossmeisl, J. K. Norskov, and K. W. Jacobsen, J. Am. Chem. Soc. 126, 13140 (2004).
C. Gatti, Z. Kristallogr. 220, 399 (2005).
V. G. Tsirelson, A. I. Stash, V. A. Potemkin, et al., Acta Crystallogr. B 62, 676 (2006).
R. F. W. Bader and H. Essen, J. Chem. Phys. 80, 1943 (1984).
C. F. Matta, N. Castillo, and R. J. Boyd, J. Phys. Chem. B 110, 563 (2006).
P. I. Dem’yanov and R. M. Gschwind, Organometallics 25, 5709 (2006).
I. Alkorta, L. Barrios, I. Rozas, et al., J. Mol. Struct. (Theochem) 496, 131 (2000).
E. Espinosa, E. Molins, and C. Lecomte, Chem. Phys. Lett. 285, 170 (1998).
S. J. Grabowski, W. A. Sokalski, E. Dyguda, et al., J. Phys. Chem. B 110, 6444 (2006).
M. V. Vener, A. V. Manaev, A. N. Egorova, et al., Khim. Fiz. 27(7), 19 (2008) [Russ. J. Phys. Chem. B 27, 512 (2008)].
W. F. Bieger-Konig, R. F. W. Bader, and T.-H. Tang, J. Comput. Chem. 3, 317 (1982).
A. I. Jiménez, G. Ballano, and C. Cativiela, Angew. Chem., Int. Ed. Engl. 44, 396 (2005).
M. Crisma, F. Formaggio, A. Moretto, et al., Biopolymers (Peptide Science) 84, 3 (2006).
R. Flaig, T. Koritsanszky, D. Dittrich, et al., J. Am. Chem. Soc. 124, 3407 (2002).
M. V. Vener, A. N. Egorova, D. P. Fomin, et al., Chem. Phys. Lett. 440, 278 (2007).
T. Steiner, Angew. Chem., Int. Ed. Engl. 41, 48 (2002).
A. N. Morosov and S. H. Lin, J. Phys. Chem. B 110, 20555 (2006).
S. Lifson and A. Roig, J. Chem. Phys. 34, 1963 (1961).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.V. Vener, A.N. Egorova, D.P. Fomin, V.G. Tsirel’son, 2009, published in Khimicheskaya Fizika, 2009, Vol. 28, No. 8, pp. 3–10.
Rights and permissions
About this article
Cite this article
Vener, M.V., Egorova, A.N., Fomin, D.P. et al. A quantum-topological analysis of noncovalent interactions in secondary polyalanine structures. Russ. J. Phys. Chem. B 3, 541–547 (2009). https://doi.org/10.1134/S1990793109040046
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990793109040046