Abstract
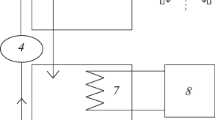
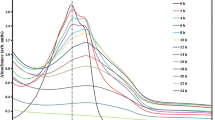
The sonochemical oxidation of phenol has been examined in airequilibrated aqueous media at various pH’s and at various insonation powers. Its disappearance follows zero-order kinetics at [phenol]initial ~ 30 to 70 μM Three principal intermediate species formed at pH 3: catechol (CC), hydroquinone (HQ), and p-benzoquinone (BQ); at natural pH (5.4–5.7) only catechol and hydroquinone formed. No intermediate species were detected at pH 12 under the conditions used. The sonochemical fate of CC, HQ, and BQ was also examined at pH 3 and at natural pH’s. At pH 3, BQ is the major species formed during insonation of HQ, while HQ is produced during insonation of BQ. In both cases, an additional intermediate formed in trace quantities that is identified as hydroxy-p-benzoquinone. These same intermediate species have been identified in the heterogeneous photocatalyzed oxidation of phenol in irradiated titania suspensions. The present results confirm the important role of ’OH radicals in degradation processes. Although CO2 is the ultimate product in heterogenous photocatalysis, irradiation of a phenolic aqueous solution by ultrasounds showed no loss of total organic carbon (TOC) after several hours, even though the aromatic substrate and the intermediates had degraded. A simple kinetic model/scheme is described to account for the events in the conversion of the substrates to products. It is concluded that the hydrophobic benzoquinone reacts with ¹OH and H¹ radicals at the hydrophobic gas bubble/liquid interface, while the hydrophilic species (phenol, CC, and HQ) react, to a large extent, with the ¹ OH radicals in the solution bulk.
Similar content being viewed by others
References
D.F. Ollis, E. Pelizzetti, and N. Serpone in Serpone N. and Pelizzetti E. (Eds.), Photocatalysis — Fundamentals and Applications, Wiley-Interscience, New York, 1989, pp. 603–637.
Y. Y. Lur’e, Russ. J. Phys. Chem., 37 (1963) 1264.
J.W. Chen, J.A. Chang, and G.V. Smith, Chem. Eng. Prog. Symp. Ser., 67 (1966) 18.
A. Kotronarou, G. Mills, and M.R. Hoffmann, J. Phys. Chem., 95 (1991) 3630.
K.S. Suslick in Suslick K.S. (Ed.), Ultrasound — Its Chemical, Physical, and Biological Effects, VCH Publishers, New York, 1988, pp. 123–163 and references therein.
M.A. Margulis and A.F. Dmitrieva, Zh. Fiz. Khim., 55 (1981) 159.
M.A. Margulis and A.F. Dmitrieva, Zh. Fiz. Khim., 56 (1982) 323; ibid, 56(1982)875.
S. Fujikawa and T.J. Akamatsu, J. Fluid Mech., 97 (1980) 481.
Both dihydrogen and dioxygen form by insonation of water in the presence of various metal catalysts and crushed glass from the secondary reactions of the radicals produced (E. Borgarello and N. Serpone, unpublished results, 1984).
P. Riesz, D. Berdahl, and C.L. Christman, Environ. Health Perspect., 64 (1985) 233.
N. Serpone, R. Terzian, C. Minero, and E. Pelizzetti, Adv. Chem. Series., 1992, in press.
H. Al-Ekabi and N. Serpone, J. Phys. Chem., 92 (1988) 5726.
K. Okamoto, Y. Yamamoto, H. Tanaka, M. Tanaka, and A. Ataya, Bull. Chem. Soc. Jpn., 58 (1985) 2015.
K. Okamoto, Y. Yamamoto, H. Tanaka, and A. Ataya, Bull. Chem. Soc. Jpn., 58 (1985) 2023.
V Augugliaro, L. Palmisano, A. Sclafani, C. Minero, and E. Pelizzetti, Toxicol. Environ. Chem., 16 (1988) 89.
R. Terzian, N. Serpone, R.B. Draper, M.A. Fox, and E. Pelizzetti, Langmuir, 7 (1991) 3081.
D. Lawless, N. Serpone, and D. Meisel, J. Phys. Chem., 95 (1991) 5166.
a) L. Zechmeister and L. Wallcave, J. Am. Chem. Soc., 77 (1955) 2953; b) L. Zechmeister and E.F. Magoon, J. Am. Chem. Soc., 78(1956)2149; c) D.L. Currell and L. Zechmeister, J. Am. Chem. Soc., 80(1958)207.
K.S. Suslick, Scientific American, 80 (1989).
Work carried out at the University of Torino.
See for example: (i) T. Kitazume, Ultrasonics, 28 (1990) 322; (ii) T. Ando and T. Kimura, Ultrasonics, 28(1990)326; (iii) A. Fadel, Tetrahedron, 47(1991)6265; (iv) G.A. Olah and A.-H. Wu, Synthesis, 135(1991)204; (v) J. Ichihara, K. Funabiki, and T. Hanafusa, Tetrahedron Lett., 31(1989)1848; (vi) B.C. Ranu and M.K. Basu, Tetrahedron Lett., 32(1991)3243; (vii) D. Goldsmith and J.J. Soria, Tetrahedrdon Lett., 32(1991)2457; and (viii) J. Berthelot, Y. Benammar, and C. Lange, Tetrahedron Lett., 32(1991)4135.
M.S. Toy and R.S. Stringham in Fox M.A. (Ed.), Organic Phototransformations in Nonhomogenous Media, ACS Symposium Series, Vol. 278, American Chemical Society, Washington, D.C., 1985, pp. 287–295.
P. Colarusso and N. Serpone, to be submitted for publication.
D.L. Currell, G. Wilheim, and S. Nagy, J. Am. Chem. Soc., 85 (1963) 127.
C.-H. Fischer, E. Hart, and A. Henglein, J. Phys. Chem., 90 (1986) 223.
J.W. Chen and G.V. Smith, Feasibility Studies of Applications of Catalytic Oxidation in Wastewater, United States Environmental Protection Agency, Report No. 17020 ECI 11/1971.
P. Riesz, T. Kondo, and C.Murali Krishna, Ultrasonics, 28 (1990) 295.
K.S. Suslick in Suslick K.S. (Ed.), High-Energy Processes in Organometallic Chemistry, American Chemical Society, Washington, D.C., 1987, Chapter 12, p. 208.
ba]E. Hart, C.-H. Fischer, and A. Henglein, J. Phys. Chem., 94 (1990) 284.
E.J. Land and M. Ebert, Trans. Faraday Soc., 63 (1967) 1181.
N. Serpone, R. Terzian, E. Pelizzetti, and H. Hidaka, to be submitted.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Serpone, N., Terzian, R., Colarusso, P. et al. Sonochemical oxidation of phenol and three of its intermediate products in aqueous media: Catechol, hydroquinone, and benzoquinone. Kinetic and mechanistic aspects. Res Chem Intermed 18, 183–202 (1993). https://doi.org/10.1163/156856792X00281
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1163/156856792X00281