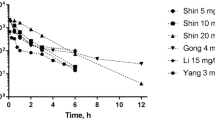
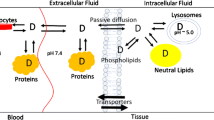
Abstract
Depending upon the drug and drug delivery platform, species-specific physiological differences can lead to errors in the interspecies extrapolation of drug performance. This manuscript provides an overview of the species-specific physiological variables that can influence the performance of parenteral dosage forms such as in situ forming delivery systems, nanoparticles, microspheres, liposomes, targeted delivery systems, lipophilic solutions, and aqueous suspensions. Also discussed are those factors that can influence the partitioning of therapeutic compounds into tumors, the central nervous system and the lymphatics. Understanding interspecies differences in the movement and absorption of molecules is important to the interpretation of data generated through the use of animal models when studying parenteral drug delivery.









Similar content being viewed by others
References
Martinez M. Interspecies differences in physiology and pharmacology: extrapolating preclinical data to human populations. In: Rogge MC, Taft DR, editors. Preclinical Drug Development. 2nd ed. Boca Raton: Taylor and Francis Group; 2009. p. 35–70.
Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos. 1995;16(5):351–80.
de Zwart LL, Rompelberg CJM, Sips AJAM, Welink J, and van Engelen JGM. Anatomical and physiological differences between various species used in studies on the pharmacokinetics and toxicology of xenobiotics. A review of literature. RIVM report 623860 010: October 1999. http://www.rivm.nl/bibliotheek/rapporten/623860010.pdf. Accessed 1 Oct 2011.
Martignoni M, Groothuis GMM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition, and induction. Expert Opin Drug Metab Toxicol. 2006;2(6):875–94.
Martinez MN, Pedersoli WM, Ravis WR, Jackson JD, Cullison R. Feasibility of interspecies extrapolation in determining the bioequivalence of animal products intended for intramuscular administration. J Vet Pharmacol Ther. 2001;24(2):125–35.
Lifschitz A, Pis A, Alvarez L, Virkel G, Sanchez S, Sallovitz J, et al. Bioequivalence of ivermectin formulations in pigs and cattle. J Vet Pharmacol Ther. 1999;22(1):27–34.
Hirano K, Yamada H. Studies on the absorption of practically water-insoluble drugs following injection VI: subcutaneous absorption from aqueous suspensions in rats. J Pharm Sci. 1982;71(5):500–5.
Larsen C, Larsen SW, Jensen H, Yaghmur A, Ostergaard J. Role of in vitro release models in formulation development and quality control of parenteral depots. Expert Opin Drug Deliv. 2009;6(12):1283–95.
Wiig H, Reed RK, Tenstad O. Interstitial fluid pressure, composition of interstitium, and interstitial exclusion of albumin in hypothyroid rats. Am J Physiol Heart Circ Physiol. 2000;278(5):H1627–39.
Schriftman H, Kondritzer AA. Absorption of atropine from muscle. Am J Physiol. 1957;191(3):591–4.
Saikawa EA, Masegi M, Hashida M, Sezaki H. Contribution of interstitial diffusion in drug absorption from perfused rabbit muscle: effect of hyaluronidase on absorption. Chem Pharm Bull (Tokyo). 1992;40(3):737–40.
Dunn AL, Heavner JE, Racz G, Day M. Hyaluronidase: a review of approved formulations, indications and off-label use in chronic pain management. Expert Opin Biol Ther. 2010;10(1):127–31.
Hanhn MS, Kobler JB, Starcher BC, Zeitels SM, Langer R. Quantitative and comparative studies of the vocal fold extracellular matrix. I: elastic fibers and hyaluronic acid. Ann Otol Rhinol Laryngol. 2006;115(2):156–64.
Laurent UB, Granath KA. The molecular weight of hyaluronate in the aqueous humour and vitreous body of rabbit and cattle eyes. Exp Eye Res. 1983;36(4):481–92.
Kuo JW. Practical aspects of hyaluronoan-based medical products. New York: CRC Press; 2006.
Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85(8):699–715.
Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4(7):528–39.
Toole BP, Slomiany MG. Hyaluronan: a constitutive regulator of chemoresistance and malignancy in cancer cells. Semin Cancer Biol. 2008;18(4):244–50.
Johnson P, Ruffell B. CD44 and its role in inflammation and inflammatory diseases. Inflamm Allergy Drug Targets. 2009;8(3):208–20.
Gee K, Kryworuchko M, Kumar A. Recent advances in the regulation of CD44 expression and its role in inflammation and autoimmune diseases. Arch Immunol Ther Exp (Warsz). 2004;52(1):13–26.
Platt VM, Szoka Jr FC. Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol Pharm. 2008;5(4):474–86.
Oh EJ, Park K, Kim KS, Kim J, Yang JA, Kong JH, et al. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J Control Release. 2010;141(1):2–12.
Eliaz RE, Szoka Jr FC. Liposome-encapsulated doxorubicin targeted to CD44: a strategy to kill CD44-overexpressing tumor cells. Cancer Res. 2001;61(6):2592–601.
Bosworth BT, St John T, Gallatin WM, Harp JA. Sequence of the bovine CD44 cDNA: comparison with human and mouse sequences. Mol Immunol. 1991;28(10):1131–5.
Nedvetzki S, Golan I, Assayag N, Gonen E, Caspi D, Gladnikoff M, et al. A mutation in a CD44 variant of inflammatory cells enhances the mitogenic interaction of FGF with its receptor. J Clin Invest. 2003;111(8):1211–20.
Skotheim RI, Nees M. Alternative splicing in cancer: noise, functional, or systematic? Int J Biochem Cell Biol. 2007;39(7–8):1432–49.
Milde KF, Alejandro R, Pastori RL. Expression of CD44 variant transcripts in dog lymphatic tissue. Immunogenetics. 1994;40(6):437–44.
Misra S, Heldin P, Hascall VC, Karamanos NK, Skandalis SS, Markwald RR, et al. Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J. 2011. doi:10.1111/j.1742-4658.2011.08071.x.
Watson PD. Analysis of the paired-tracer method of determining cell uptake. Am J Physiol. 1998;275(2 Pt 1):E366–71.
Hollenstein UM, Brunner M, Schmid R, Müller M. Soft tissue concentrations of ciprofloxacin in obese and lean subjects following weight-adjusted dosing. Int J Obes Relat Metab Disord. 2001;25(3):354–8.
Schmittmann G, Rohr UD. Comparison of the permeability surface product (PS) of the blood capillary wall in skeletal muscle tissue of various species and in vitro porous membranes using hydrophilic drugs. J Pharm Sci. 2000;89(1):115–27.
Porter CJ, Edwards GA, Charman SA. Lymphatic transport of proteins after s.c. injection: implications of animal model selection. Adv Drug Deliv Rev. 2001;50(1–2):157–71.
Edwards GA, Porter CJ, Caliph SM, Khoo SM, Charman WN. Animal models for the study of intestinal lymphatic drug transport. Adv Drug Deliv Rev. 2001;50(1–2):45–60.
Kagan L, Gershkovich P, Mendelman A, Amsili S, Ezov N, Hoffman A. The role of the lymphatic system in subcutaneous absorption of macromolecules in the rat model. Eur J Pharm Biopharm. 2007;67(3):759–65.
Oussoren C, Zuidema J, Crommelin DJA, Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. I. Influence of the anatomical site of injection. J Liposome Res. 1997;7(1):85–99.
Oussoren C, Storm G. Liposomes to target the lymphatics by subcutaneous administration. Adv Drug Deliv Rev. 2001;50(1–2):143–56.
Kota J, Machavaram KK, McLennan DN, Edwards GA, Porter CJ, Charman SA. Lymphatic absorption of subcutaneously administered proteins: influence of different injection sites on the absorption of darbepoetin alfa using a sheep model. Drug Metab Dispos. 2007;35(12):2211–7.
Bleuel H, Hoffmann R, Kaufmann B, Neubert P, Ochlich PP, Schaumann W. Kinetics of subcutaneous versus intravenous epoetin-beta in dogs, rats and mice. Pharmacology. 1996;52(5):329–38.
Woo S, Jusko WJ. Interspecies comparisons of pharmacokinetics and pharmacodynamics of recombinant human erythropoietin. Drug Metab Dispos. 2007;35(9):1672–8.
Caldini A, Moneti G, Fanelli A, Bruschettini A, Mercurio S, Pieraccini G, et al. Epoetin alpha, epoetin beta and darbepoetin alfa: two-dimensional gel electrophoresis isoforms characterization and massspectrometry analysis. Proteomics. 2003;3(6):937–41.
McLennan DN, Porter CJH, Edwards GA, Martin SW, Heatherington AC, Charman SA. Lymphatic absorption is the primary contributor to the systemic availability of epoetin alfa following subcutaneous administration to sheep. J Pharmacol Exp Ther. 2005;313(1):345–51.
Plum A, Agerso H, Andersen L. Pharmacokinetics of the rapid-acting insulin analog, insulin aspart, in rats, dogs, and pigs, and pharmacodynamics of insulin aspart in pigs. Drug Metab Dispos. 2000;28(2):155–60.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10(7):1093–5.
Brocks DR, Ramaswamy M, MacInnes AI, Wasan KM. The stereoselective distribution of halofantrine enantiomers within human, dog, and rat plasma lipoproteins. Pharm Res. 2000;17(4):427–31.
Kosa T, Maruyama T, Sakai N, Yonemura N, Yahara S, Otagiri M. Species differences of serum albumins: I: drug binding sites. Pharm Res. 1998;14(11):1607–12.
Fura A, Harper TW, Zhang H, Fung L, Shyu WC. Shift in pH of biological fluids during storage and processing: effect on bioanalysis. J Pharm Biomed Anal. 2003;32(3):513–22.
Kochansky CJ, McMasters DR, Lu P, Koeplinger KA, Kerr HH, Shou M, et al. Impact of pH on plasma protein binding in equilibrium dialysis. Mol Pharm. 2008;5(3):438–48.
Pistolozzi M, Bertucci C. Species-dependent stereoselective drug binding to albumin: a circular dichronism study. Chirality. 2008;20(3–4):552–8.
Kurtzhals P, Havelund S, Jonassen I, Kiehr B, Ribel U, Markussen J. Albumin binding and time action of acylated insulins in various species. J Pharm Sci. 1996;85(3):304–8.
Basken NE, Mathias CJ, Lipka AE, Green MA. Species dependence of [64Cu]Cu–Bis(thiosemicarbazone) radiopharmaceutical binding to serum albumins. Nucl Med Biol. 2008;35(3):281–6.
Giron MC. Radiopharmaceutical pharmacokinetics in animals: critical considerations. Q J Nucl Med Mol Imaging. 2009;53(4):359–64.
Puigdemont A, Arboix M, Gaspari F, Bortolotti A, Bonati M. In-vitro plasma protein binding of propafenone and protein profile in eight mammalian species. Res Commun Chem Pathol Pharmacol. 1989;64(3):435–40.
Lin JH, Chen IW, Deluna FA. Nonlinear kinetics of aldronate. Plasma protein binding and bone uptake. Drug Metab Disp. 1994;22(3):400–5.
Bonapace CR, Fowler S, Laessig KA, Lazor JA, Nambiar S. Ceftriaxone and calcium-containing fluids-rationale for product label changes, abstr. A1-008. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. Washington: American Society for Microbiology; 2009.
Wasan KM, Brocks DR, Lee SD, Sachs-Barrable K, Thornton SJ. Impact of lipoproteins on the biological activity and disposition of hydrophobic drugs: implications for drug discovery. Nat Rev Drug Discov. 2008;7(1):84–99.
Ramaswamy M, Wallace TL, Cossum PA, Wasan KM. Species differences in the proportion of plasma lipoprotein lipid carried by high-density lipoproteins influence the distribution of free and liposomal nystatin in human, dog, and rat plasma. Antimicrob Agents Chemother. 1999;43(6):1424–8.
Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob Agents Chemother. 2002;46(3):828–33.
Hong Y, Shaw PJ, Tattam BN, Nath CE, Earl JW, Stephen KR, et al. Plasma protein distribution and its impact on pharmacokinetics of liposomal amphotericin B in paediatric patients with malignant diseases. Eur J Clin Pharmacol. 2007;63(2):165–72.
Jin Y, Ai P, Xin R, Tian Y, Dong J, Chen D, et al. Self-assembled drug delivery systems: Part 3. In vitro/in vivo studies of the self-assembled nanoparticulates of cholesteryl acyl didanosine. Int J Pharm. 2009;368(1–2):207–14.
Jemal M, Hawthorne DJ. Quantitative determination of BMS-186716, a thiol compound, in rat plasma by high-performance liquid chromatography-positive ion electrospray mass spectrometry after hydrolysis of the methyl acrylate adduct by the native esterases. J Chromatogr B: Biomed Sci Appl. 1997;698(1–2):123–32.
Minagawa T, Kohno Y, Suwa T, Tsuji A. Species differences in hydrolysis of isocarbacyclin methyl ester (TEI-9090) by blood esterases. Biochem Pharmacol. 1995;49(10):1361–5.
Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol Pharm. 2008;5(4):487–95.
Haley PJ. Species differences in the structure and function of the immune system. Toxicology. 2003;188(1):49–71.
Brinks V, Jiskoot W, Schellekens H. Immunogenicity of therapeutic proteins: the use of animal models. Pharm Res. 2011;28(10):2379–85.
Ishida T, Harashima H, Kiwada H. Interactions of liposomes with cells in vitro and in vivo: opsonins and receptors. Curr Drug Metab. 2001;2(4):397–409.
Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307(1):93–102.
Moghimi SM, Hunter AC. Recognition by macrophages and liver cells of opsonized phospholipid vesicles and phospholipid headgroups. Pharm Res. 2001;18(1):1–8.
Yan X, Scherphof GL, Kamps JA. Liposome opsonization. J Liposome Res. 2005;15(1–2):109–39.
Liu D, Hu Q, Song YK, Song. Liposome clearance from blood: different animal species have different mechanisms. Biochemica et Biophysica Acta. 1995;1240(2):277–84.
Huong TM, Harashima H, Kiwada H. In vivo studies on the role of complement in the clearance of liposomes in rats and guinea pigs. Biol Pharm Bull. 1999;22(5):515–20.
Kurihara A, Shibayama Y, Kasuya A, Ikeda M, Hisaoka M. Species variation in pharmacokinetics and opsonization of palmitoyl rhizoxin (RS-1541) incorporated in lipid emulsions. J Drug Target. 1998;5(6):491–505.
Szebeni J. Complement activation-related pseudoallergy caused by amphiphilic drug carriers: the role of lipoproteins. Curr Drug Deliv. 2005;2(4):443–9.
Szebeni J, Alving CR, Rosivall L, Bünger R, Baranyi L, Bedöcs P, et al. Animal models of complement-mediated hypersensitivity reactions to liposomes and other lipid-based nanoparticles. J Liposome Res. 2007;17(2):107–17.
Liu S, Ishida T, Kiwada H. Effect of serum components from different species on destabilizing hydrogenated phosphatidylcholine-based liposomes. Biol Pharm Bull. 1997;20(8):874–80.
Harashima H, Komatsu S, Kojima S, Yanagi C, Morioka Y, Naito M, et al. Species difference in the disposition of liposomes among mice, rats, and rabbits: allometric relationship and species dependent hepatic uptake mechanism. Pharm Res. 1996;13(7):1049–54.
Matschkea C, Iselea U, van Hoogevestb P, Fahrc A. Sustained-release injectables formed in situ and their potential use for veterinary products. J Control Release. 2002;85(103):1–15.
Packhaeuser CB, Schnieders J, Oster CG, Kissel T. In situ forming parenteral drug delivery systems: an overview. Eur J Pharm Biopharm. 2004;58(2):445–55.
Leppert PS, Cammack L, Cargill R, Coffman L, Cortese M, Engle K, et al. Interspecies differences in systemic drug availability following subcutaneous pulsatile administration in cattle, sheep, dogs, and rats. J Biomed Mater Res. 1994;28(6):713–22.
Ali MJ, Navalitloha Y, Vavra MW, Kang EW, Itskovich AC, Molnar P, et al. Isolation of drug delivery from drug effect: problems of optimizing drug delivery parameters. Neuro Oncol. 2006;8(2):109–18.
Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47(12):3039–51.
Netti PA, Hamberg LM, Babich JW, Kierstead D, Graham W, Hunter GJ, et al. Enhancement of fluid filtration across tumor vessels: implication for delivery of macromolecules. Proc Natl Acad Sci U S A. 1999;96(6):3137–42.
Stohrer M, Boucher Y, Stangassinger M, Jain RK. Oncotic pressure in solid tumors is elevated. Cancer Res. 2000;60(15):4251–5.
Thomas LA, Brown SA. Relationship between colloid osmotic pressure and plasma protein concentration in cattle, horses, dogs, and cats. Am J Vet Res. 1992;53(12):2241–4.
Zweifach BW, Intaglietta M. Measurement of blood plasma colloid osmotic pressure: II. Comparative study of different species. Microvasc Res. 1971;3(1):83–8.
Navar PD, Navar LG. Relationship between colloid osmotic pressure and plasma protein concentration in the dog. Am J Physiol. 1977;233(2):H295–8.
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13(4):407–84.
Chang PL. Non-autologous somatic gene therapy. In: Chang PL, editor. Somatic gene therapy. Boca Raton: CRC Press, Inc; 1995. p. 203–24.
Ross CJD, Ralph M, Chang PL. Somatic gene therapy for a neurodegenerative disease using microencapsulated recombinant cells. Exp Neurol. 2000;166(2):276–86.
Gagliardi C, Bunnell BA. Large animal models of neurological disorders for gene therapy. ILAR J. 2009;50(2):128–43.
Barsoum SC, Milgram W, Mackay W, Coblentz C, Delaney KH, Kwiecien JM, et al. Delivery of recombinant gene product to canine brain with the use of microencapsulation. J Lab Clin Med. 2003;142(6):399–413.
Shull R, Lu X, Dube I, Lutzko C, Kruth S, Abrams-Oqq A, et al. Humoral immune response limits gene therapy in canine MPS I. Blood. 1996;88(1):377–9.
Shull RM, Lu X, McEntee MF, Bright RM, Pepper KA, Kohn DB. Myoblast gene therapy in canine mucopolysaccharidosis. I. Abrogation by an immune response to alpha-L-iduronidase. Hum Gene Ther. 1996;7(13):1595–603.
Aebischer P, Goddard M, Signore AP, Timpson RL. Functional recovery in hemiparkinsonian primates transplanted with polymer-encapsulated PC12 cells. Exp Neurol. 1994;126(2):151–8.
Aebischer P, Schluep M, Déglon N, Joseph JM, Hirt L, Heyd B, et al. Intrathecal delivery of CNTF using encapsulated genetically modified xenogeneic cells in amyotrophic lateral sclerosis patients. Nat Med. 1996;2(6):696–9.
Bloch J, Bachoud-Lévi AC, Déglon N, Lefaucheur JP, Winkel L, Palfi S, et al. Neuroprotective gene therapy for Huntington's disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: results of a phase I study. Hum Gene Ther. 2004;15(10):968–75.
Kawakami J, Yamamoto K, Sawada Y, Iga T. Prediction of brain delivery of ofloxacin, a new quinolone, in the human from animal data. J Pharmacokinet Biopharm. 1994;22(3):207–27.
Smith EK, Gustafson EL, Borden LA, Dhar TGM, Durkin MM, Vaysse PJJ, et al. Heterogeneity of brain GABA transporters. In: Tanaka C, Bowery NG, editors. GABA: receptors, transporters, and metabolism. Switzerland: Birkauser Verlag; 1996. p. 63–72.
Sharif NA, Nunes JL, Michel AD, Whiting RL. Comparative properties of the dopamine transport complex in dog and rodent brain: striatal [(3)H]GBR12935 binding and [(3)H]dopamine uptake. Neurochem Int. 1989;15(3):325–32.
Gerhart DZ, Leino RL, Borson ND, Taylor WE, Gronlund KM, McCall AL, et al. Localization of glucose transporter GLUT 3 in brain: comparison of rodent and dog using species-specific carboxyl-terminal antisera. Neuroscience. 1995;66(1):237–46.
Mantych GJ, James DE, Chung HD, Devaskar SU. Cellular localization and characterization of Glut 3 glucose transporter isoform in human brain. Endocrinology. 1992;131(3):1270–8.
Westerhout J, Danhof M, De Lange EC. Preclinical prediction of human brain target site concentrations: considerations in extrapolating to the clinical setting. J Pharm Sci. 2011. doi:10.1002/jps.
Warren MS, Zerangue N, Woodford K, Roberts LM, Tate EH, Feng B, et al. Comparative gene expression profiles of ABC transporters in brain microvessel, endothelial cells and brain in five species including human. Pharmacol Res. 2009;59(6):404–13.
Baltes S, Gastens AM, Fedrowitz M, Potschka H, Kaever V, Löscher W. Differences in the transport of the antiepileptic drugs phenytoin, levetiracetam and carbamazepine by human and mouse P-glycoprotein. Neuropharmacology. 2007;52(2):333–46.
Syvänen S, Lindhe O, Palner M, Kornum BR, Rahman O, Långström B, et al. Species differences in blood-brain barrier transport of three positron emission tomography radioligands with emphasis on P-glycoprotein transport. Drug Metab Dispos. 2009;37(3):635–43.
Liu X, Chen C, Smith BJ. Progress in brain penetration evaluation in drug discovery and development. J Pharm Exp Ther. 2008;325(2):349–56.
Reiber H, Thiele P. Species-dependent variables in blood cerebrospinal fluid barrier function for proteins. J Clin Chem Clin Biochem. 1983;21(4):199–202.
Dhuria SV, Hanson LR, Frey 2nd WH. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–73.
Liu CH, You Z, Ren JQ, Kim YR, Eikermann-Haerter K, Liu PK. Noninvasive delivery of gene targeting probes to live brains for transcription MRI. FASEB J. 2008;22(4):1193–203.
Di L, Umland JP, Chang G, Huang Y, Lin Z, Scott DO, et al. Species independence in brain tissue binding using brain homogenates. Drug Metab Dispos. 2011;39(7):1270–7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Marilyn Martinez
Rights and permissions
About this article
Cite this article
Martinez, M.N. Factors Influencing the Use and Interpretation of Animal Models in the Development of Parenteral Drug Delivery Systems. AAPS J 13, 632–649 (2011). https://doi.org/10.1208/s12248-011-9303-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-011-9303-8