Abstract
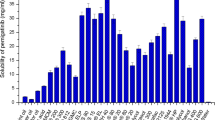
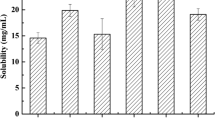
The purpose of this research work was to formulate and characterize self-micro emulsifying drug delivery system containing exemestane. The solubility of exemestane was determined in various vehicles. Pseudo ternary phase diagram was used to evaluate the micro-emulsification existence area. SMEDDS formulations were tested for micro-emulsifying properties, and the resultant formulations loaded with exemestane (ME1, ME2, ME3, ME4 and ME5) were investigated for clarity, phase separation, globule size and shape, zeta potential, effect of various diluents and dilutions, thermodynamic and thermal stability. From the results it is concluded that increase in droplet size is proportional to the concentration of oil in SMEDDS formulation. Minor difference in the droplet size and zeta potential was observed by varying the diluents (deionized water and 0.1 N HCl) and dilutions (1:10, 1:50 and 1:100). Formulations, which were found to be thermodynamically stable (ME1, ME2, ME3 and ME4), were subjected to stability studies as per International Conference on Harmonization (ICH) guidelines. No significant variations were observed in the formulations over a period of 3 months at accelerated and long-term conditions. TEM photographs of microemulsions formulations further conformed the spherical shape of globules. Among the various SMEDDS formulations, ME4 offer the advantages of good clarity systems at high oil content and thus offer good solubilization of exemestane. Thus this study indicates that the SMEDDS can be used as a potential drug carrier for dissolution enhancement of exemestane and other lipophilic drug(s).




Similar content being viewed by others
References
J. R. Robinson. Introduction: semi-solid formulations for oral drug delivery. Bull. Tech.-Gattefosse. 89:11–13 (1996).
B. J. Aungst. Novel formulation strategies for improving oral bioavailability of drugs with poor membrane permeation or presystemic metabolism. J. Pharm. Sci. 82:979–987 (1993).
A. J. Humberstone, and W. N. Charman. Lipid-based vehicles for the oral delivery of poorly water-soluble drugs. Adv. Drug Del. Rev. 25:103–128 (1997).
W. L. Chiou, S. J. Chen, and N. Athanikar. Enhancement of dissolution rates of poorly water-soluble drugs by crystallization in aqueous surface solutions. I. Sulfathiazole, prednisone, and chloramphenicol. J. Pharm. Sci. 65:1702–1704 (1976).
C. W. Pouton. Self-emulsifying drug delivery systems: assessment of the efficiency of emulsification. Int. J. Pharm. 27:335–348 (1985).
C. W. Pouton. Effects of the inclusion of a model drug on the performance self-emulsifying formulations. J. Pharm. Pharmacol. 37:1P (1985).
C. W. Pouton. Formulation of self-emulsifying drug delivery systems. Adv. Drug Deliv. Rev. 25:47–58 (1997).
T. T. Kararli, T. E. Needham, M. Grifaen, G. Schoenhard, L. J. Ferro, and L. Alcorn. Oral delivery of a rennin inhibitor compound using emulsion formulation. Pharm. Res. 9:888–893 (1992).
R. A. Schwendener, and H. Schott. Lipophilic 1-beta-d-arabinofuranosyl cytosine derivatives in liposomal formulations for oral and parenteral antileukemic therapy in the murine L1210 leukemia model. J. Cancer Res. Clin. Oncol. 122:723–726 (1996).
H. Shen, and M. Zhong. Prepration and evaluation of self-microemulsifying drug delivery systems (SMEDDS) containing atorvastatin. J. Pharm. Pharmacol. 58:1183–1191 (2006).
D. K. Wang, Z. H. Shi, L. Liu, X. Y. Wang, C. X. Zhang, and P. Zhao. Development of Self-microemulsifying drug delivery systems for oral bioavailability enhancement of a-Asarone in beagle dogs. PDA J. Pharm. Sci. Tech. 60(6):343–349 (2006).
P. P. Constantinides. Lipid microemulsion for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm. Res. 12(11):1561–1572 (1995).
P. K. Ghosh, and R. S. R. Murthy. Microemulsions: a potential drug delivery system. Curr. Drug Deliv. 3:167–180 (2006).
P. E. Lonning. Pharmacological profiles of exemestane and formestane, steroidal aromatase inhibitors used for the treatment of post-menopausal breast cancer. Breast Can. Res. Treat. 49:S45–S52 (1998).
K. S. Weippl, and P. E. Goss. Prevention of breast cancer using SERMs and aromatase inhibitors. J. Mammary Gland Biol. Neoplasia. 8:5–18 (2003).
M. Dowsett. Theoretical considerations for the ideal aromatase inhibitors. Breast Can. Res. Treat. 49:S39–S44 (1998).
Physician Desk Reference. Thomson Healthcare, Montvale, NJ. 60th edition, 2006, pp. 2600–2602.
B. K. Kang, J. S. Lee, S. K. Chon, S. Y. Jeong, S. H. Yuk, G. Khang, H. B. Lee, and S. H. Cho. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int. J. Pharm. 274:65–73 (2004).
T. Lucas, R. Bishara, and R. Seevers. A stability program for the distribution of drug products. Pharma. Tech. 68–71 (2004).
T. R. Kommuru, B. Gurley, M. A. Khan, and I. K. Reddy. Self-emulsifying drug delivery systems (SEDDS) of co-enzyme Q10: formulation development and bioavailability assessment. Int. J. Pharm. 212:233–246 (2001).
P. P. Constantinides, C. M. Lancaster, and J. Marcello. Enhanced intestinal absorption of an RGD peptide from water-in-oil microemulsions of different composition and particle size. J. Control. Release. 34:109–116 (1995).
Acknowledgement
The authors would like to thank Gattefosse (Saint Priest, France) and GPS Pharma (Delhi, India) for providing the excipients for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, A.K., Chaurasiya, A., Singh, M. et al. Exemestane Loaded Self-Microemulsifying Drug Delivery System (SMEDDS): Development and Optimization. AAPS PharmSciTech 9, 628–634 (2008). https://doi.org/10.1208/s12249-008-9080-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-008-9080-6