Abstract
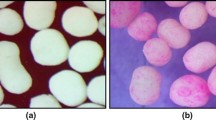
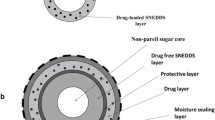
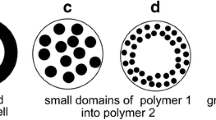
Conventionally, scanning electron or transmission microscopy, Raman and near infrared (NIR) spectroscopy, terahertz, florescence, and nuclear magnetic resonance imaging have been used to characterize functional coating structure. This study highlights the use of fluorescence microscopy to investigate the physicochemical stability and coating integrity of the commercially available enteric-coated omeprazole pellets containing a basic excipient and prepared by extrusion and spheronization or drug layering on the nonpareil seed, immediately followed by enteric coating (i.e., absence of protective sub-coat). The nature of coating interface and the likely development of an in situ interfacial layer after the application of enteric coating solution was examined using HPLC, NMR, differential scanning calorimetry (DSC), and fluorescent imaging methods. Likewise for the characterization of the solid pellet structure via fluorescence microscopy, a new approach based on fracturing technique (to avoid surface contamination) rather than microtome sectioning was used and validated. Analytical data showed that the pellets containing omeprazole remained chemically stable (>99.5% recovered). Control of the microenvironmental pH by the addition of alkalinizing excipient within a core formulation or as part of drug layering on top of nonpareil seed appears to efficiently neutralize the acidic effect of enteric coating dispersion. Fluorescence images further illustrate the absence of any discernable in situ layer formation at the coat-core interface.









Similar content being viewed by others
References
Robinson M. Proton pump inhibitors: update on their role in acid-related gastrointestinal diseases. Int J Clin Pract. 2005;59:709–15.
Hershcovici T, Fass R. Pharmacological management of GERD: where does it stand now? Trends Pharmacol Sci. 2011;32:258–64.
Lindberg P, Brandstrom A, Wallmark B. Structure-activity-relationships of omeprazole analogs and their mechanism of action. Trends Pharmacol Sci. 1987;8:399–402.
Lind T, Cederberg C, Ekenved G, Haglund U, Olbe L. Effect of omeprazole-a gastric proton pump inhibitor-on pentagastrin stimulated acid secretion in man. Gut. 1983;24:270–6.
Pilbrant A, Cederberg C. Development of an oral formulation of omeprazole. Scand J Gastroenterol. 1985;20:113–20.
Thoma K, Bechtold K. Enteric coated hard gelatin capsules. Capsugel Libr. 2000;145:1–17.
Pillay V, Fassihi R. In vitro release modulation from crosslinked pellets for site-specific drug delivery to the gastrointestinal tract. I. Comparison of pH-responsive drug release and associated kinetics. J Control Release. 1999;59:229–42.
Kolte BP, Tele KV, Mundhe VS, Lahoti SS. Colon targeted drug delivery system—a novel perspective. Asian J Biomed Pharm Sci. 2012;2:21–8.
Mathew M, Das Gupta V, Bailey RE. Stability of omeprazole solutions at various pH values as determined by HPLC. Drug Dev Ind Pharm. 1995;21:965–71.
Missaghi S. Formulation design and approaches to enteric coating delivery via compression coating or encapsulation of acid-labile compounds using omeprazole as a model drug. PhD dissertation, Temple University, School of Pharmacy; Philadelphia, 2006. Chapter 4.
Erickson M, Josefson L. Pharmaceutical formulation of omeprazole. 1988. US Patent 6090827.
Chen CM, Chou J, Weng T. Omeprazole formulation. 1999. US Patent 6077541.
Chen CM, Chou J, Weng T. Omeprazole formulation. 1999. US Patent 6096340.
Stroyer A, McGinity JW, Leopold CS. Solid state interactions between the proton pump inhibitor omeprazole and various enteric coating polymers. J Pharm Sci. 2006;95:1342–53.
Lundberg PJ, Lovgren K. New pharmaceutical formulation and process. 1996. WO96/24338.
Lee FY, Chen SC, Kuo HC. Orally administered pharmaceutical formulations of benzimidazole derivatives and method of preparing the same. 2000. US Patent 6228400B1.
Jantzen GM, Robinson GR. Sustained-and controlled-release drug-delivery systems. In: Banker GS, Rhodes CT, editors. Modern pharmaceutics. 4th ed. New York: Marcel Dekker; 2002. p. 501–28.
Porter SC. Coating of pharmaceutical dosage forms. In: Gennaro AR, editor. Remington, the science and practice of pharmacy, 20th Edition: Lippincott Williams and Wilkins; 2000. p. 894–902.
Munday DL, Fassihi AR. Controlled release delivery: effect of coating composition on release characteristics of mini-tablets. Int J Pharm. 1989;52:109–14.
Coutts-Lendon CA, Wright NA, Mieso EV. The use of FT-IR imaging as an analytical tool for characterization of drug delivery systems. J Control Release. 2003;93:223–48.
Missaghi S, Fassihi AR. A novel approach in the assessment of polymeric film formation and film adhesion on different pharmaceutical solid substrates. AAPS Pharm Sci Tech. 2004;5(2):1–8.
Merrett K, Cornelius RM, McClung WG, Unsworth LD, Sheardown H. Surface analysis methods for characterizing polymeric biomaterials. J Biomater Sci Polym Ed. 2002;13:593–621.
Munday DL, Fassihi AR, De Villiers C. Bioavailability study of a theophylline oral controlled release capsule containing film coated mini-tablets in beagle dogs. Int J Pharm. 1991;69:123–7.
Munday DL, Fassihi AR. Changes in drug release rate: effect of stress storage conditions on film coated mini-tablets. Drug Dev Ind Pharm. 1991;17:2135–43.
Dave K, Scozzafava A, Vullo D, Supuran CT, Ilies MA. Pyridinium derivatives of histamine are potent activators of cytosolic carbonic anhydrase isoforms I, II and VII. Org Biomol Chem. 2011;9:2790–800.
Savarala S, Brailoiu E, Wunder SL, Ilies MA. Tuning the self-assembling of pyridinium cationic lipids for efficient gene delivery into neuronal cells. Biomacromolecules. 2013;14(8):2750–64.
Sharma VD, Aifuwa EO, Heiney PA, Ilies MA. Interfacial engineering of pyridinium gemini surfactants for the generation of synthetic transfection systems. Biomaterials. 2013;34(28):6906–21.
US Pharmacopeia, 25th Edition: USP Convention Inc; 2002. p. 1265–6.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sharma, V.D., Akocak, S., Ilies, M.A. et al. Solid-State Interactions at the Core-Coat Interface: Physicochemical Characterization of Enteric-Coated Omeprazole Pellets Without a Protective Sub-Coat. AAPS PharmSciTech 16, 934–943 (2015). https://doi.org/10.1208/s12249-014-0263-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0263-z