Abstract
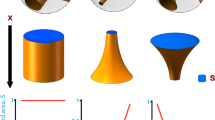
Development of new product design principles is crucial for obtaining pharmaceutical products with controlled functionality. Four different molds were designed using a computer-aided design (CAD) software and 3D printed with polylactic acid (PLA). A hydroxypropyl methylcellulose (HPMC) and polyethylene glycol (PEG)–based formulation containing indomethacin as the active pharmaceutical ingredient (API) was casted into the molds. Each mold produced a tablet that was designed to disintegrate into a defined number of sections (2, 4, and 6). This was achieved by incorporating break lines (regions that were significantly thinner than the remainder of the tablet) to control the disintegration process. Disintegration and drug release from these designed tablets was contrasted with a casted tablet without break lines. Disintegration studies confirmed that the casted tablets disintegrated according to their design. Drug-release studies meanwhile demonstrated that tablets with a greater number of sections released the API at a faster rate than those with fewer sections; for example, the 6-sectioned tablet released the API at twice the rate of the tablet without any break lines. It is expected that by using this concept, it would be possible to produce tablets with a designed disintegration profile, which could potentially allow the tailoring of the drug release.






Similar content being viewed by others
References
Cohen JS. Dose discrepancies between the physicians’ desk reference and the medical literature: and their possible role in the high incidence of dose-related adverse drug events. Arch Intern Med. 2001;161(7):957–64.
Cohen JS. Ways to minimize adverse drug reactions. Postgrad Med. 1999;106(3):163–72.
van der Hooft CS, Sturkenboom MCJM, van Grootheest K, Kingma HJ, Stricker BHC. Adverse drug reaction-related hospitalisations: a nationwide study in The Netherlands. Drug Saf. 2006 [cited 2018 Jul 20];29(2):161–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16454543. Accessed 22 Feb 2019.
Alomari M, Mohamed FH, Basit AW, Gaisford S. Personalised dosing: printing a dose of one’s own medicine. Int J Pharm. 2015;494(2):568–77. Available from: https://doi.org/10.1016/j.ijpharm.2014.12.006.
Crommelin DJA, Bouwman-Boer Y. Pharmacy preparations: back in the limelight? Pharmacists make up your mind! Int J Pharm. 2016 [cited 2017 Jun 16];514(1):11–4. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0378517316308523. Accessed 22 Feb 2019.
Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005 [cited 2018 Jul 20];352(21):2211–21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15917386. Accessed 22 Feb 2019.
Sastry SV, Nyshadham JR, Fix JA. Recent technological advances in oral drug delivery–a review. Pharm Sci Technol Today. 2000 [cited 2018 Jul 20];3(4):138–45. Available from: https://www.sciencedirect.com/science/article/pii/S1461534700002479. Accessed 22 Feb 2019.
Alhnan MA, Okwuosa TC, Sadia M, Wan KW, Ahmed W, Arafat B. Emergence of 3D printed dosage forms: opportunities and challenges. Pharm Res. 2016;33(8):1817–32. Available from: https://doi.org/10.1007/s11095-016-1933-1.
Al-khattawi A, Mohammed AR. Challenges and emerging solutions in the development of compressed orally disintegrating tablets. Expert Opin Drug Discov. 2014 [cited 2018 Jul 20];9(10):1109–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25045997. Accessed 22 Feb 2019.
Goyanes A, Buanz ABM, Basit AW, Gaisford S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int J Pharm. 2014;476(1):88–92.
Berman B. 3-D printing: the new industrial revolution. Bus Horiz. 2012 [cited 2018 Jul 20];55(2):155–62. Available from: https://www.sciencedirect.com/science/article/pii/S0007681311001790. Accessed 22 Feb 2019.
Katstra W, Palazzolo R, Rowe C, Giritlioglu B, Teung P, Cima M. Oral dosage forms fabricated by three dimensional printing™. J Control Release. 2000;66(1):1–9.
Markarian J. Using 3D printing for solid-dosage drugs. [cited 2018 Jul 20]; Available from: http://www.pharmtech.com/using-3d-printing-solid-dosage-drugs. Accessed 22 Feb 2019.
Goyanes A, Det-amornrat U, Wang J, Basit AW, Gaisford S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J Control Release. 2016;234:41–8. Available from: https://doi.org/10.1016/j.jconrel.2016.05.034.
Melocchi A, Parietti F, Maroni A, Foppoli A, Gazzaniga A, Zema L. Hot-melt extruded filaments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int J Pharm. 2016;509(1):255–63.
McMains S Sara. Layered manufacturing technologies. Commun ACM. 2005 [cited 2018 Jul 20];48(6):50. Available from: http://portal.acm.org/citation.cfm?doid=1064830.1064858. Accessed 22 Feb 2019.
Lim SH, Kathuria H, Tan JJY, Kang L. 3D printed drug delivery and testing systems—a passing fad or the future? Adv Drug Deliv Rev. 2018 [cited 2018 Jul 20]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/29778901. Accessed 22 Feb 2019.
Goyanes A, Robles P, Buanz A, Basit AW, Gaisford S. Effect of geometry on drug release from 3D printed tablets. Int J Pharm. 2015;494(2):657–63. Available from: https://doi.org/10.1016/j.ijpharm.2015.04.069.
Goyanes A, Buanz ABM, Hatton GB, Gaisford S, Basit AW. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur J Pharm Biopharm. 2015 [cited 2018 Jul 20];89:157–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25497178. Accessed 22 Feb 2019.
Kyobula M, Adedeji A, Alexander MR, Saleh E, Wildman R, Ashcroft I, et al. 3D inkjet printing of tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J Control Release. 2017 [cited 2017 Aug 7];261:207–15. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0168365917306892. Accessed 22 Feb 2019.
Sadia M, Arafat B, Ahmed W, Forbes RT, Alhnan MA. Channelled tablets: an innovative approach to accelerating drug release from 3D printed tablets. J Control Release. 2018 [cited 2018 Jul 20];269:355–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29146240. Accessed 22 Feb 2019.
Arafat B, Wojsz M, Isreb A, Forbes RT, Isreb M, Ahmed W, et al. Tablet fragmentation without a disintegrant: a novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur J Pharm Sci. 2018 [cited 2018 Jul 20];118:191–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29559404. Accessed 22 Feb 2019.
Goyanes A, Wang J, Buanz A, Telford R, Gaisford S, Basit AW. 3D printing of medicines: engineering novel oral devices with unique design and drug release characteristics. Mol Pharm. 2015;12:4077–88.
Norman J, Madurawe RD, Moore CMV, Khan MA, Khairuzzaman A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv Drug Deliv Rev. 2016.
Sun Y, Soh S. Printing tablets with fully customizable release profiles for personalized medicine. Adv Mater. 2015 [cited 2016 Oct 19];27(47):7847–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26498272. Accessed 22 Feb 2019.
Bellinger AM, Jafari M, Grant TM, Zhang S, Slater HC, Wenger EA, et al. Oral, ultra–long-lasting drug delivery: application toward malaria elimination goals. Sci Transl Med. 2016 [cited 2017 Jun 7];8(365):365ra157. Available from: http://stm.sciencemag.org/content/8/365/365ra157. Accessed 22 Feb 2019.
Lim L-T, Auras R, Rubino M. Processing technologies for poly(lactic acid). Prog Polym Sci. 2008 [cited 2018 Jul 20];33(8):820–52. Available from: https://www.sciencedirect.com/science/article/pii/S0079670008000373. Accessed 22 Feb 2019.
Wittbrodt B, Pearce JM. The effects of PLA color on material properties of 3-D printed components. Addit Manuf. 2015 [cited 2018 Jul 20];8:110–6. Available from: https://www.sciencedirect.com/science/article/pii/S2214860415000494. Accessed 22 Feb 2019.
Yu LX. Pharmaceutical quality by design: product and process development, understanding, and control. Pharm Res. 2008 [cited 2018 Jul 20];25(4):781–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18185986. Accessed 22 Feb 2019.
Shakeel F, Alanazi FK, Alsarra IA, Haq N. Solubility prediction of indomethacin in PEG 400 + water mixtures at various temperatures. J Mol Liq. 2013 [cited 2018 Jul 20];188:28–32. Available from: https://www.sciencedirect.com/science/article/pii/S0167732213002961. Accessed 22 Feb 2019.
Vraníková B, Gajdziok J, Doležel P. The effect of superdisintegrants on the properties and dissolution profiles of liquisolid tablets containing rosuvastatin. Pharm Dev Technol. 2017 [cited 2018 Jul 20];22(2):138–47. Available from: https://www.tandfonline.com/doi/full/10.3109/10837450.2015.1089900. Accessed 22 Feb 2019.
Quodbach J, Kleinebudde P. A critical review on tablet disintegration. Pharm Dev Technol. 2015 [cited 2018 Jul 20];21(6):1–12. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25975586. Accessed 22 Feb 2019.
Kabiri K, Omidian H, Hashemi SA, Zohuriaan-Mehr MJ. Synthesis of fast-swelling superabsorbent hydrogels: effect of crosslinker type and concentration on porosity and absorption rate. Eur Polym J. 2003 [cited 2018 Jul 20];39(7):1341–8. Available from: https://www.sciencedirect.com/science/article/pii/S0014305702003919. Accessed 22 Feb 2019.
Zhang B, Wang K, Si J, Sui M, Shen Y. Charge-reversal polymers for biodelivery. In: Bioinspired and biomimetic polymer systems for drug and gene delivery. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2014 [cited 2019 Jan 9]. p. 223–42. Available from: http://doi.wiley.com/10.1002/9783527672752.ch9.
Funding
This work was supported by the Independent Research Fund Denmark (IRFD), Technology and Production Sciences (FTP), grant number 12-126515/0602-02670B. Cosima Hirschberg acknowledges Innovation Fund Denmark (Project- High Quality Dry Products with Superior Functionality and Stability–Q-Dry; File No: 5150-00024B) for funding.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editors: Niklas Sandler and Jukka Rantanen
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ajmal, A., Meskarzadeh, A., Genina, N. et al. The Use of 3D Printed Molds to Cast Tablets with a Designed Disintegration Profile. AAPS PharmSciTech 20, 127 (2019). https://doi.org/10.1208/s12249-019-1341-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1341-z