Abstract
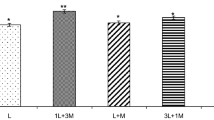
Myocardial infarction (MI) is the principal cause of death in many countries. Silymarin (SM) is a herbal antioxidant and can be efficiently used in preventing cardiovascular diseases (CVDs). The study is aimed to enhance the absorption rate and biological activity of SM by using liquisolids besides investigating the cardioprotective activity of SM and its selected liquisolid formula against isoproterenol prompted cardiotoxicity in rats. Eight formulae were prepared according to (23) full-factorial design. The effect of viscosity increasing agent type and concentration, as well as the carrier/coat ratio on the dissolution rate and angle of repose were studied. All formulae were tested for content uniformity, micromeritic properties, dissolution performance besides the evaluation of its physicochemical properties, and scanning electron microscopy (SEM). Based on the factorial design outcomes, the highest desirability was obtained from F3 with excipient ratio value (R) of 20%, dissolution rate at Q5 min of 26.9%, and angle of repose of 19. Oral administration of F3 liquisolid and SM revealed a significant protective efficacy against the modification of cardiac plasma markers, brain natriuretic peptide (BNP), interleukin-10 (IL-10), vascular endothelial growth factor (VEGF), and transforming growth factor (TGF)-β1 besides cardiac superoxide dismutase (SOD), malondialdehyde (MDA), and total protein kinase-1 (Akt-1) levels. Additionally, they minimized cardiac inducible nitric oxide synthase (iNOS), microRNA-34a (miR-34a), and p38 mitogen-activated protein kinase (p38-MAPK) levels. In conclusion, F3 liquisolid compact possessed an overall pronounced results over pure SM reckoned to its enhanced solubility and efficacy.











Similar content being viewed by others
References
Yousaf AM, Malik UR, Shahzad Y, Mahmood T, Hussain T. Silymarin-laden PVP-PEG polymeric composite for enhanced aqueous solubility and dissolution rate: preparation and in vitro characterization. J Pharm Anal. 2019;9:34–9.
Sornsuvit C, Hongwiset D, Yotsawimonwat S, Toonkum M, Thongsawat S, Taesotikul W. The bioavailability and pharmacokinetics of silymarin SMEDDS formulation study in healthy Thai volunteers. Evid Based Complement Alternat Med. 2018;1–7.
Javed S, Kohli K, Ali M. Reassessing bioavailability of silymarin. Alternative Medicine Rev. 2011;16:239–49.
Taleb A, Ahmad KA, Ihsan AU, Qu J, Lin N, Hezam K, et al. Antioxidant effects and mechanism of silymarin in oxidative stress induced cardiovascular diseases. Biomed Pharmacother. 2018;102:689–98.
Surai PF. Silymarin as a natural antioxidant: an overview of the current evidence and perspectives. Antioxidants (Basel). 2015;4(1):204–47.
Rahimia R, Karimia J, Khodadadia I, Tayebiniaa H, Kheiripourb N, Hashemniac M, et al. Silymarin ameliorates expression of urotensin II (U-II) and its receptor (UTR) and attenuates toxic oxidative stress in the heart of rats with type 2 diabetes. Biomed Pharmacother. 2018;101:244–50.
Chen L, Hu JY, Wang SQ. The role of antioxidants in photoprotection: a critical review. J Am Acad Dermatol. 2012;23:231–40.
Alcantara EH, Shin MY, Sohn HY, Park YM, Kim T, Lim JH, et al. Indole alkaloids from Catharanthusroseus: bioproduction and their effect on human health. Molecules. 2015;20:2973–3000.
Eleni T, Kateřina P, Haralambos S, Fragiskos K, Vladimír K. Bioavailability of silymarin flavonolignans: drug formulations and biotransformation. Phytochem Rev. 2014;13:1–18.
Di Costanzo A, Angelico R. Formulation strategies for enhancing the bioavailability of silymarin: the state of the art. Molecules. 2019;24(11):2155.
Piazzini V, D’Ambrosio M, Luceri C, Cinci L, Landucci E, Bilia AR, et al. Formulation of nanomicelles to improve the solubility and the oral absorption of silymarin. Molecules. 2019;24:1688.
Lu M, Ding P. Liquisolid technique and its applications in pharmaceutics. Asian J Pharm Sci. 2017;12:115–23.
Javadzadeh Y, Jafari-Navimipour B, Nokhodchi A. Liquisolid technique for dissolution rate enhancement of a high dose water-insoluble drug (carbamazepine). Int J Pharm. 2007;341:26–34.
Soliman SM, Abdel Malak NS, El-Gazayerly ON, Abdel Rehim AA. Formulation of microemulsion gel systems for transdermal delivery of celecoxib: in vitro permeation, anti-inflammatory activity and skin irritation tests. Drug Discov Ther. 2010;4(6):459–71.
Balata GF. Formulation and evaluation of gliclazide in vegetable oil-based self emulsifying delivery system. J App Pharm Sci. 2018;8(09):023–33.
Sonali D, Tejal S, Vaishali T, Tejal G. Silymarin-solid dispersions: characterization and influence of preparation methods on dissolution. Acta Pharma. 2010;60:427–43.
Naveen C, Shastri N, Tadikond RR. Use of the liquisolid compact technique for improvement of the dissolution rate of valsartan. Acta Pharma Sin B. 2012;2:502–8.
Swain S, Niranjan C, Bhanoji Rao ME. Pharmaceutical drug delivery systems and vehicles. 1st ed. Woodhead Publishing India: Publisher; 2015.
Pavani E, Noman S, Syed IA. Liquisolid technique based sustained release tablet of trimetazidine dihydrochloride. Drug invention today. 2013;5:302–10.
Han X, Qi W, Dong W, Guo M, Ma P, Wang J. Preparation, optimization and in vitro–in vivo investigation for capsules of the choline salt of febuxostat. Asian J Pharm Sci. 2016;11:715–21.
Pharmacopoeia B. The stationery office. MD: Rockville; 2012.
Kamel R, Basha M. Preparation and in vitro evaluation of Rutin nanostructured liquisolid delivery system. Bulletin of Faculty of Pharmacy, Cairo University. 2013;51:261–72.
Panda A, Biyani DM. Studies on liquisolid system as a technique to modify the dissolution rate of nifedipine. Am J Pharmtech Res. 2013;3:686–98.
Mahmoud EA, Bendas ER, Mohamed MI. Preparation and evaluation of self-nanoemulsifying tablets of carvedilol. AAPS PharmSciTech. 2009;10:183–92.
Javadzadeh Y, Siahi MR, Asnaashari S, Nokhodchi A. An investigation of physicochemical properties of piroxicam liquisolid compacts. Pharm Dev Tech. 2008;12:337–43.
Patil JS, Devi VK, Devi K, Sarasija S. A novel approach for lung delivery of rifampicin-loaded liposomes in dry powder form for the treatment of tuberculosis. Lung India. 2015;32(4):331–8.
Yang G, Zhao Y, Feng N, Zhang Y, Liu Y, Dang B. Improved dissolution and bioavailability of silymarin delivered by a solid dispersion prepared using supercritical fluids. Asian J Pharm Sci. 2015;10:194–202.
Reema O, Chandrasekhar S, Chandrakala K. Bio-tea prevents membrane destabilization during isoproterenol-induced myocardial injury. J Microsc Ultrastruct. 2017;5:146–54.
Farah N, Nasim J, Nayma S, Mohammad H. Effects of peanut (Arachis hypogaea L.) and its combination with propranolol in isoproterenol induced myocardial damage in rats. J Bangladesh Soc Physiol. 2016;11:74–9.
Gerhardt W, Waldenström J. Creatine kinase B-subunit activity in serum after immunoinhibition of M-subunit activity. Clin Chem. 1979;25:1274–80.
King J. The dehydrogenase or oxidoreductatse. Lactate dehydrogenase. In: Practical clinical enzymology. London: Nostr. and Co.; 1965.
Nichans WH, Samulelson B. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–30.
Marklund S, Marklund D. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74.
Hentzschel CM, Sakmann A, Leopold CS. Suitability of various excipients as carrier and coating materials for liquisolid compacts. Drug Dev Ind Pharm. 2011;37:1200–7.
Jadhav NR, Pharm M, Irny PV, Patil US. Solid state behavior of progesterone and its release from Neusilin US2 based liquisolid compacts. J Drug Deliv Sci Tech. 2017;38:97–106.
Hill SW, Varker AS, Karlage K, Myrdal PB. Analysis of drug content and weight uniformity for half-tablets of 6 commonly split medications. J Manag Care Pharm. 2009;15(3):253–61.
Baranauskaite J, Kopustinskiene DM, Masteikova R, Gajdziok J, Baranauskas A, Bernatoniene J. Effect of liquid vehicles on the enhancement of rosmarinic acid and carvacrol release from oregano extract liquisolid compacts. Colloids Surf A Physicochem Eng Asp. 2018;539:280–90.
Nokhodchi A, Aliakbar R, Desai S, Javadzadeh Y. Liquisolid compacts: the effect of cosolvent and HPMC on theophylline release. Colloids Surfaces B Biointerfaces. 2010;79:262–9.
Tiong N, Elkordy AA. Effects of liquisolid formulations on dissolution of naproxen. Eur J Pharm Biopharm. 2009;73:373–84.
Tayel SA, Soliman II, Louis D. Improvement of dissolution properties of carbamazepine through application of the liquisolid tablet technique. Eur J Pharm Biopharm. 2008;69:342–7.
Jaipakdee N, Limpongsa E, Sripanidkulchai B, Piyachaturawat P. Preparation of Curcuma comosa tablets using liquisolid techniques: in vitro and in vivo evaluation. Int J Pharm. 2018;553:157–68.
Sharma V, Pathak K. Effect of hydrogen bond formation/replacement on solubility characteristics, gastric permeation and pharmacokinetics of curcumin by application of powder solution technology. Acta Pharma Sin B. 2016;6:600–13.
Spireas S, Wang T, Grover R. Effect of powder substrate on the dissolution properties of methyclothiazide liquisolid compacts. Drug Dev Ind Pharm. 1999;25:163–8.
Komala DR, Janga KY, Jukanti R, Bandari S, Gopa MV. Competence of raloxifene hydrochloride loaded liquisolid compacts for improved dissolution and intestinal permeation. J Drug Deliv Sci Tech. 2015;30:232–41.
Elkadi S, Elsamaligy S, Al-Suwayeh S, Mahmoud H. The development of self-nanoemulsifying liquisolid tablets to improve the dissolution of simvastatin. AAPS PharmSciTech. 2017;18:2586–97.
Barmpalexis P, Grypioti A, Eleftheriadis GK, Fatouros DG. Development of a new aprepitant liquisolid formulation with the aid of artificial neural networks and genetic programming. AAPS PharmSciTech. 2018;19:741–52.
Varshosaz J, Tavakoli N, Kheirolahi F. Use of hydrophilic natural gums in formulation of sustained-release matrix tablets of tramadol hydrochloride. AAPS PharmSciTech. 2006;7(1):E1–7.
Xu H, Liu L, Li X, Ma J, Liu R, Wang S. Extended tacrolimus release via the combination of lipid-based solid dispersion and HPMC hydrogel matrix tablets. Asian J Pharm Sci. 2019;14:445–54.
Elkordy AA, Essa EA, Dhuppad S, Jammigumpula P. Liquisolid technique to enhance and to sustain griseofulvin dissolution: effect of choice of non-volatile liquid vehicles. Int J Pharm. 2012;434:122–32.
Ghosh A, Biswas S, Ghosh T. Preparation and evaluation of silymarin b-cyclodextrin molecular inclusion complexes. J Young Pharmacists. 2011;3:205–10.
Suryawanshi VK, Gidwani B, Verma A, Dubey N, Kaur CD. Formulation and evaluation of ramipril liquisolid compact using novel carrier. Int J Pharm Sci Res. 2019;10:917–25.
Noor AD, Al-Khedairy EBH. Formulation and evaluation of silymarin as microcrystals by in- situ micronization technique. Iraqi J Pharm Sci. 2019;28(1):1–16.
Lian R, Lu Y, Qi J, Tan Y, Niu M, Guan P, et al. Silymarin glyceryl monooleate/poloxamer 407 liquid crystalline matrices: physical characterization and enhanced oral bioavailability. AAPS PharmSciTech. 2011;12:1234–40.
Morazzoni P, Bombardelli E. Silybum marianum (Carduus marianus). Fitoterapia. 1995;66:3–42.
Haková H, Misúrová E, Kropácová K. The effect of silymarin on concentration and total content of nucleic acids in tissues of continuously irradiated rats. Vet Med (Praha). 1996;41:113–9.
Kang JS, Jeon YJ, Park SK, Yang KH, Kim HM. Protection against lipopolysaccharide-induced sepsis and inhibition of interleukin-1beta and prostaglandin E2 synthesis by silymarin. Biochem Pharmacol. 2004;67:175–81.
Wang Q, Zou L, Liu W, Hao W, Tashiro S, Onodera S, et al. Inhibiting NF-κB activation and ROS production are involved in the mechanism of silibinin’s protection against d-galactose-induced senescence. Pharmacol Biochem Behav. 2011;98:140–9.
Prabu SM, Muthumani M. Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Mol Biol Rep. 2012;39:11201–16.
Wang C, Wang Z, Zhang X, Zhang X, Dong L, Xing Y, et al. Protection by silibinin against experimental ischemic stroke: up-regulated pAkt, pmTOR, HIF-1α and Bcl-2, down-regulated Bax, NF-κB expression. Neurosci Lett. 2012;529:45–50.
Liu BN, Hanm BX, Liu F. Neuroprotective effect of pAkt and HIF-1 α on ischemia rats. Asian Pac J Trop Med. 2014;7:221–5.
Aristatile B, Al-Assaf AH, Pugalendi KV. Carvacrol suppresses the expression of inflammatory marker genes in d-galactosamine-hepatotoxic rats. Asian Pac J Trop Med. 2013;6:205–11.
Perrelli MG, Pagliaro P, Penna C. Ischemia/ reperfusion injury and cardioprotective mechanisms: role of mitochondria and reactive oxygen species. World J Cardiol. 2011;3:186–200.
Zhou B, Wu LJ, Tashiro S, Onodera S, Uchiumi F, Ikejima T. Silibinin protects rat cardiac myocyte from isoproterenol-induced DNA damage independent on regulation of cell cycle. Biol Pharm Bull. 2006;29:1900–5.
Rolo AP, Oliveira PJ, Moreno AJ, Palmeira CM. Protection against post-ischemic mitochondrial injury in rat liver by silymarin or TUDC. Hepatol Res. 2003;26:217–24.
Detaille D, Sanchez C, Sanz N, Lopez-Novoa JM, Leverve X, El-Mir MY. Interrelation between the inhibition of glycolytic flux by silibinin and the lowering of mitochondrial ROS production in perifused rat hepatocytes. Life Sci. 2008;82:1070–6.
Owona BA, Njayou NF, Laufer SA, Schluesener HJ, Moundipa PF. Entada africana fraction CH(2)cl(2)/MEOH 5% inhibits inducible nitric oxide synthase and pro-inflammatory cytokines gene expression induced by lipopolysaccharide in microglia. BMC Complement Altern Med. 2013;13:254.
Zeng L, Tang WJ, Yin JJ, Zhou BJ. Signal transductions and nonalcoholic fatty liver: a mini-review. Int J Clin Exper Med. 2014;7:1624–31.
Luo C, Yang H, Tang C. Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. Int Immunopharmacol. 2015;28:744–50.
Cordero-Herrera I, Martin MA, Goya L, Ramos S. Cocoa flavonoids protect hepatic cells against high-glucose-induced oxidative stress: relevance of MAPKs. Mol Nutr Food Res. 2015;59:597–609.
Xu ZR, Li JY, Dong XW. Apple polyphenols decrease atherosclerosis and hepatic steatosis in ApoE−/− mice through the ROS/MAPK/NF-κB pathway. Nutrients. 2015;7:7085–105.
Usta E, Mustafi M, Walker T, Ziemer G. Resveratrol suppresses apoptosis in intact human cardiac tissue - in vitro model simulating extracorporeal circulation. J Cardiovasc Surg. 2011;52:399–409.
Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–72.
Huang Z, Wu S, Kong F, Cai X, Ye B. MicroRNA-21 protects against cardiac hypoxia/ reoxygenation injury by inhibiting excessive autophagy in H9c2 cells via the Akt/mTOR pathway. J Cell Mol Med. 2017;21:467–74.
Shao H, Yang L, Wang L, Tang B, Wang J. MicroRNA-34a protect myocardial cells against ischemiareperfusion injury through inhibiting autophagy via regu- lating TNF-α expression. Biochem Cell Biol. 2018;96:349–54.
Vegter EL, van der Meer P, de Windt LJ, Pinto YM, Voors AA. MicroRNAs in heart failure: from biomarker to target for therapy. Eur J Heart Fail. 2016;18:457–68.
Lee YC, Cheng TH, Lee JS, Chen JH, Liao YC, Fong Y, et al. Nobiletin, a citrus flavonoid, suppresses invasion and migration involving FAK/PI3K/Akt and small GTPase signals in human gastric adenocarcinoma AGS cells. Mol Cell Biochem. 2011;347:103–15.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were performed in accordance with guidelines established by the animal care and use approved by research ethical committee of O6U, Faculty of Pharmacy, Egypt.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sheta, N.M., Elfeky, Y.A. & Boshra, S.A. Cardioprotective Efficacy of Silymarin Liquisolid in Isoproterenol Prompted Myocardial Infarction in Rats. AAPS PharmSciTech 21, 81 (2020). https://doi.org/10.1208/s12249-019-1609-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1208/s12249-019-1609-3