Abstract
Background
The intraoperative epidural analgesia (EA) has the potential to reduce stress response to surgical trauma which induces a transient immunoactivation that has a negative impact on the outcome. This study investigates the effect of intraoperative EA versus intravenous analgesia (IA) on the immune function.
Methods
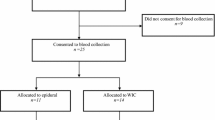
A total of 35 consecutive patients candidated to undergo major surgery for colon cancer were randomly assigned to intraoperative EA (n = 18) or IA (n = 17). Blood samples for TNF-α, IFN-γ, IL-1, IL-2, IL-4, IL-6, IL-10, IL-12, and GM-CSF were obtained before surgery (Tpre), 3 h (T3h), and 24 h (T24h) after skin incision. Data on postoperative complications were prospectively collected and analyzed.
Results
In the EA group, IL-4 increased from Tpre to T3h and from T3h to T24h, IL-10 increased from Tpre to T3h and persisted unmodified thereafter. At all time-points, IL-4 and IL-10 serum levels were significantly higher than those in the IA group. Conversely, in the IA group, IL-4 and IL-10 serum levels did not change while all other cytokines levels were significantly higher compared with the EA group. In particular, IL-6 progressively reached a 7-fold increase of its basal value at T24h. Complications were significantly more common in IA patients (13 of 17) compared with EA patients (7 of 18) (P = .024).
Conclusions
Our results indicate that in cancer patients undergoing major elective colon surgery, the EA attenuates the surgery-induced proinflammatory response and the typical postoperative transient immunosuppression and seems associated with a reduced rate of postoperative complications compared with IA.




Similar content being viewed by others
References
Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–17.
Dunn AJ. Interaction between the nervous system and the immune system. In: Dunn AJ, ed. Psychopharmacology—The Fourth Generation of Progress. New York: Raven Press.
Ishikawa M, Nishioka M, Hanaki N, Miyauchi T, Kashiwagi Y, Ioki H, et al. Perioperative immune responses in cancer patients undergoing digestive surgeries. World J Surg Oncol. 2009;12;7:7.
Haupt W, Riese J, Mehler C, Weber K, Zowe M, Hohenberger W. Monocyte function before and after surgical trauma. Dig Surg. 1998;15:102–4.
Kato M, Suzuki H, Murakami M, Akama M, Matsukawa S, Hashimoto Y. Elevated plasma levels of interleukin-6, interleukin-8, and granulocyte colony-stimulating factor during and after major abdominal surgery. J Clin Anesth. 1997;9:293–8.
Sheeran P, Hall GM. Cytokines in anaesthesia. Br J Anaesth. 1997;78:201–19.
McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug Alcohol Depend. 2001;62:111–23.
Sacerdote P. Opioid-induced immunosuppression. Curr Opin Support Palliat Care. 2008;2:14–8.
Stefano GB, Fricchione G, Goumon Y, Esch T. Pain, immunity, opiate and opioid compounds and health. Med Sci Monit. 2005;11:MS47–53.
Hall DM, Suo JL, Weber RJ. Opioid mediated effects on the immune system: sympathetic nervous system involvement. J Neuroimmunol. 1998;83:29–35.
Shimizu H, Ito H, Kimura F, Togawa A, Togawa A, Yoshidome H, Ohtsuka M, et al. Decreased cell-mediated immune status in colorectal cancer patients with hepatic metastasis. Hepatogastroenterol. 2005;52:1106–9.
Heriot AG, Marriott JB, Cookson S, Kumar D, Dalgleish AG. Reduction in cytokine production in colorectal cancer patients: association with stage and reversal by resection. Br J Cancer. 2000;82:1009–12.
Kurosawa S, Kato M. Anesthetics, immune cells, and immune responses. J Anesth. 2008;22:263–77.
Kelbel I, Weiss M. Anaesthetics and immune function. Curr Opin Anaesthesiol. 2001;14:685–9.
Angele MK, Faist E. Clinical review: immunodepression in the surgical patient and increased susceptibility to infection. Crit Care. 2002;6:298–305.
Helmy SA, Wahby MA, El-Nawaway M. The effect of anaesthesia and surgery on plasma cytokine production. Anaesthesia. 1999;54:733–8.
Buunen M, Gholghesaei M, Veldkamp R, Meijer DW, Bonjer HJ, Bouvy ND. Stress response to laparoscopic surgery: a review. Surg Endosc. 2004;18:1022–8.
Moraca RJ Sheldon DG, Thirlby RC. The role of epidural anaesthesia and analgesia in surgical practice. Ann Surg. 2003;238:663–73.
Rigg JR, Jamrozik K, Myles PS, Silbert BS, Peyton PJ, Parsons RW, et al. MASTER Anaesthesia Trial Study Group. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359:1276–82.
Procopio MA, Rassias AJ, DeLeo JA, Pahl J, Hildebrandt L, Yeager MP. The in vivo effects of general and epidural anesthesia on human immune function. Anesth Analg. 2001;93:460–5.
Ahlers O, Nachtigall I, Lenze J, Goldmann A, Schulte E, Höhne C, et al. Intraoperative thoracic epidural anaesthesia attenuates stress-induced immunosuppression in patients undergoing major abdominal surgery. Br J Anaesth. 2008;101:781–7.
Kim C, Sakamoto A. Differences in the leukocyte response to incision during upper abdominal surgery with epidural versus general anesthesia. J Nippon Med Sch. 2006;73:4–9.
Valién JL, Hernando AN, Gascón AH, Francés RC, Lobera JC. [Cellular immune response to 3 anesthetic techniques for simple abdominal hysterectomy]. Rev Esp Anesthesiol Reanim. 2007;54:523–8 [in Spanish].
Yokoyama M, Itano Y, Katayama H, Morimatsu H, Takeda Y, Takahashi T, et al. The effects of continuous epidural anesthesia and analgesia on stress response and immune function in patients undergoing radical esophagectomy. Anesth Analg. 2005;101:1521–7.
Kawasaki T, Ogata M, Kawasaki C, Okamoto K, Sata T. Effects of epidural anaesthesia on surgical stress-induced immunosuppression during upper abdominal surgery. Br J Anaesth. 2007;98:196–203.
Norman JG, Fink GW. The effects of epidural anesthesia on the neuroendocrine response to major surgical stress: a randomized prospective trial. Am Surg. 1997;63:75–80.
Taniguchi T, Koido Y, Aiboshi J, Yamashita T, Suzaki S, Kurokawa A. Change in the ratio of interleukin-6 to interleukin-10 predicts a poor outcome in patients with systemic inflammatory response syndrome. Crit Care Med. 1999;27:1262–4.
Dindo D, Demartines N, Clavien P. Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163:316–21.
Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–8.
Opal SM, DePalo VA. Anti-Inflammatory cytokines. Chest. 2000;117:1162–7.
Szczesny TJ Slotwinski R, Stankiewicz A, Szczygiel B, Zaleska M, Kopacz M. Interleukin 6 and interleukin 1 receptor antagonist as early markers of complications after lung cancer surgery. Eur L Cardiothorac Surg. 2007;31:719–24.
Baker EA, El-Gaddal S, Williams L, Leaper DJ. Profiles of inflammatory cytokines following colorectal surgery: relationship with wound healing and outcome. Wound Repair Regen. 2006;14:566–72.
Hietbrink F, Koenderman L, Rijkers G, Leenen L. Trauma: the role of the innate immune system. World J Emerg Surg. 2006;1:15.
Sander M, von Heymann C, von Dossow V, Spaethe C, Konertz WF, Jain U, et al. Increased interleukin-6 after cardiac surgery predicts infection. Anesth Analg. 2006;102:1623–9.
Tashiro T, Yamamori H, Takagi K, Hayashi N, Furukawa K, Nitta H, et al. Changes in immune function following surgery for esophageal carcinoma. Nutrition. 1999;15:760–6.
Watkins LR, Maier SF, Goehler LE. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63:289–302.
Murphy GS Szokol JW, Marymont JH, Avram MJ, Vender JS. The effects of morphine and fentanyl on the inflammatory response to cardiopulmonary bypass in patients undergoing elective coronary artery bypass graft surgery. Anesth Analg. 2007;104:1334–42.
Homburger JA, Meiler SE. Anesthesia drugs, immunity, and long-term outcome. Curr Opin Anaesthesiol. 2006;19:423–8.
Borgdorff PJ, Ionescu TI, Houweling PL, Knape JT. Large-dose intrathecal sufentanil prevents the hormonal stress response during major abdominal surgery: a comparison with intravenous sufentanil in a prospective randomized trial. Anesth Analg. 2004;99:1114–20.
Akural EI, Salomäki TE, Bloigu AH, Ryhänen P, Tekay AH, Alahuhta SM, et al. The effects of pre-emptive epidural sufentanil on human immune function. Acta Anaesthesiol Scand. 2004;48:750–5.
Beilin B, Bessler H, Mayburd E, Smirnov G, Dekel A, Yardeni I, et al. Effects of preemptive analgesia on pain and cytokine production in the postoperative period. Anesthesiology. 2003;98:151–5.
Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–63.
Oberholzer A, Oberholzer C, Moldawer LL. Interleukin-10: a complex role in the pathogenesis of sepsis syndromes and its potential as an anti-inflammatory drug. Crit Care Med. 2002;30:S58–63.
Oberholzer A, Souza SM, Tschoeke SK, Oberholzer C, Abouhamze A, Pribble JP, et al. Plasma cytokine measurements augment prognostic scores as indicators of outcome in patients with severe sepsis. Shock. 2005;23:488–93.
Latifi SQ, O’Riordan MA, Levine AD. Interleukin-10 controls the onset of irreversible septic shock. Infect Immun. 2002;70:4441–6.
van Dissel JT, van Langevelde P, Westendorp RG, Kwappenberg K, Frölich M. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet. 1998;351:950–3.
Heper Y, Akalin EH, Mistik R, Akgöz S, Töre O, Göral G, et al. Evaluation of serum C-reactive protein, procalcitonin, tumor necrosis factor alpha, and interleukin-10 levels as diagnostic and prognostic parameters in patients with community-acquired sepsis, severe sepsis, and septic shock. Eur J Clin Microbiol Infect Dis. 2006;25:481–91.
Kragsbjerg P, Holmberg; Vikefors T. Serum concentrations of interleukin-6, tumour necrosis factor-alpha, and C-reactive protein in patients undergoing major operations. Eur J Surg. 1995;161:17–22.
Holmes JH 4th, Connolly NC, Paull DL, Hill ME, Guyton SW, Ziegler SF, et al. Magnitude of the inflammatory response to cardiopulmonary bypass and its relation to adverse clinical outcomes. Inflamm Res. 2002;51:579–86.
Ishida K, Kimura F, Imamaki M, Ishida A, Shimura H, Kohno H, et al. Relation of inflammatory cytokines to atrial fibrillation after off-pump coronary artery bypass grafting. Eur J Cardiothorac Surg. 2006;29:501–5.
Ucar HI, Tok M, Atalar E, Dogan OF, Oc M, Farsak B, et al. Predictive significance of plasma levels of interleukin-6 and high-sensitivity C-reactive protein in atrial fibrillation after coronary artery bypass surgery. Heart Surg Forum. 2007;10:E131–5.
Gueret G, Lion F, Guriec N, Arvieux J, Dovergne A, Guennegan C, et al. Acute renal dysfunction after cardiac surgery with cardiopulmonary bypass is associated with plasmatic IL6 increase. Cytokine. 2009;45:92–8.
Laurent C, Sa Cunha A, Couderc P, Couderc P, Rullier E, Saric J. Influence of postoperative morbidity on long-term survival following liver resection for colorectal metastases. Br J Surg. 2003;90:1131–6.
Mizuguchi T, Nagayama M, Meguro M, Shibata T, Kaji S, Nobuoka T, et al. Prognostic impact of surgical complications and preoperative serum hepatocyte growth factor in hepatocellular carcinoma patients after initial hepatectomy. J Gastrointest Surg. 2009;13:325–33.
Acknowledgment
The authors wish to thank Ms. Sarah Capper for her editorial assistance during manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moselli, N.M., Baricocchi, E., Ribero, D. et al. Intraoperative Epidural Analgesia Prevents the Early Proinflammatory Response to Surgical Trauma. Results from a Prospective Randomized Clinical Trial of Intraoperative Epidural Versus General Analgesia. Ann Surg Oncol 18, 2722–2731 (2011). https://doi.org/10.1245/s10434-011-1700-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-011-1700-9