Abstract
Background
The staging of pancreatic neuroendocrine tumors (PNETs) is continuously evolving. Mitotic count, as measured by hematoxylin and eosin (H&E) or Ki67 labeling index (Ki67LI), is the best predictor of disease biology. However, both of these methods have several limitations. Phosphorylated histone H3 (PHH3), a novel mitotic marker, is potentially more accurate and easier to evaluate. This study aimed to evaluate the prognostic impact of PHH3 on patients with PNETs.
Methods
Clinicopathologic data and paraffin-embedded tissue were evaluated for 100 of the 247 PNET patients whose tumors were resected between 1998 and 2010. Mitotic counts were analyzed on H&E-, Ki67-, and PHH3-stained slides by two independent pathologists. Kaplan–Meier curves, log-rank tests, Cox regression models, and time-dependent receiver operative characteristics (ROC) curves were used to evaluate the prognostic power of these markers. An internal data cross-validation was performed to select the best cutoff.
Results
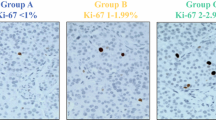
Of the 100 PNET patients resected, 53 were men. The median age of the patients was 59 years (range 19–96 years). The median follow-up period was 68 months (range 3–186 months). The median time for evaluation of an H&E- or PHH3-stained slide was 3 min, relative to 15 min for Ki67. The findings showed H&E, Ki67, and PHH3 all to be excellent predictors of disease-specific survival (DSS). However, PHH3 was superior to H&E and Ki67 in predicting both disease-free survival (DFS) (p = 0.006) and DSS (p = 0.001). Evaluation of the PHH3 mitotic count showed 7 mitoses per 10 high-power fields (HPFs) to be the optimal cutoff for differentiating between low- and high-risk PNET patients.
Conclusions
PHH3 is a better predictor of both DFS and DSS than H&E or Ki67 in PNET. In addition, PHH3 appears to be both easier to interpret and more accurate when compared to current prognostic markers.



Similar content being viewed by others
References
Franko J, Feng W, Yip L, Genovese E, Moser AJ. Nonfunctional neuroendocrine carcinoma of the pancreas: incidence, tumor biology, and outcomes in 2158 patients. J Gastrointest Surg. 2010;14:541–8.
Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metabol Clin N Am. 2011;40:1–18, vii.
Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis, and recent trend toward improved survival. Ann Oncol. 2008;19:1727–33.
de Wilde RF, Edil BH, Hruban RH, Maitra A. Well-differentiated pancreatic neuroendocrine tumors: from genetics to therapy. Nat Rev. 2012;9:199–208.
Kuo JH, Lee JA, Chabot JA. Nonfunctional pancreatic neuroendocrine tumors. Surg Clin N Am. 2014;94:689–708.
Liu TC, Hamilton N, Hawkins W, Gao F, Cao D. Comparison of WHO Classifications (2004, 2010), the Hochwald grading system, and AJCC and ENETS staging systems in predicting prognosis in locoregional well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2013;37:853–9.
Bosman FT, Carneiro F, Hruban RH, Theise N. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC Press; 2010.
Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (eds). AJCC cancer staging manual. New York: Springer; 2010. pp. 241–9.
Rindi G, Falconi M, Klersy C, Albarello L, Boninsegna L, Buchler MW, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst. 2012;104:764–77.
McCall CM, Shi C, Cornish TC, Klimstra DS, Tang LH, Basturk O, et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am J Surg Pathol. 2013;37:1671–7.
Yang Z, Tang LH, Klimstra DS. Gastroenteropancreatic neuroendocrine neoplasms: historical context and current issues. Semin Diagn Pathol. 2013;30:186–96.
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–5.
Wei Y, Mizzen CA, Cook RG, Gorovsky MA, Allis CD. Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc Natl Acad Sci USA. 1998;95:7480–4.
Ribalta T, McCutcheon IE, Aldape KD, Bruner JM, Fuller GN. The mitosis-specific antibody anti-phosphohistone-H3 (PHH3) facilitates rapid reliable grading of meningiomas according to WHO 2000 criteria. Am J Surg Pathol. 2004;28:1532–6.
Colman H, Giannini C, Huang L, Gonzalez J, Hess K, Bruner J, et al. Assessment and prognostic significance of mitotic index using the mitosis marker phospho-histone H3 in low- and intermediate-grade infiltrating astrocytomas. Am J Surg Pathol. 2006;30:657–64.
Casper DJ, Ross KI, Messina JL, Sondak VK, Bodden CN, McCardle TW, et al. Use of anti-phosphohistone H3 immunohistochemistry to determine mitotic rate in thin melanoma. Am J Dermatopathol. 2010;32:650–4.
Nasr MR, El-Zammar O. Comparison of pHH3, Ki-67, and survivin immunoreactivity in benign and malignant melanocytic lesions. Am J Dermatopathol. 2008;30:117–22.
Tsuta K, Raso MG, Kalhor N, Liu DD, Wistuba, II, Moran CA. Histologic features of low- and intermediate-grade neuroendocrine carcinoma (typical and atypical carcinoid tumors) of the lung. Lung Cancer Amst Neth. 2011;71:34–41.
Voss SM, Riley MP, Lokhandwala PM, Wang M, Yang Z. Mitotic count by phosphohistone H3 immunohistochemical staining predicts survival and improves interobserver reproducibility in well-differentiated neuroendocrine tumors of the pancreas. Am J Surg Pathol. 2015;39:13–24.
Ogino T, Bandoh N, Hayashi T, Miyokawa N, Harabuchi Y, Ferrone S. Association of tapasin and HLA class I antigen down-regulation in primary maxillary sinus squamous cell carcinoma lesions with reduced survival of patients. Clin Cancer Res. 2003;9:4043–51.
Neuroendocrine tumors. NCCN Clinical Practical Guidelines in Oncology (NCCN Guidelines) version 12015. National Comprehensive Cancer Network, 2014.
Scarpa A, Mantovani W, Capelli P, Beghelli S, Boninsegna L, Bettini R, et al. Pancreatic endocrine tumors: improved TNM staging and histopathologic grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23:824–33.
Gullo L, Migliori M, Falconi M, Pederzoli P, Bettini R, Casadei R, et al. Nonfunctioning pancreatic endocrine tumors: a multicenter clinical study. Am J Gastroenterol. 2003;98:2435–9.
Bilimoria KY, Talamonti MS, Tomlinson JS, Stewart AK, Winchester DP, Ko CY, et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg. 2008;247(3):490–500.
Acknowledgment
The Loeffler Family and other generous patients provided philanthropic support for this study. Matteo Ligorio is the recipient of American-Italian Cancer Foundation Post-Doctoral Fellowship. Vincenzo Villani is the recipient of a Research Fellowship from the Centro per la Comunicazione e la Ricerca of the Collegio Ghislieri of Pavia.
Conflicts of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Vincenzo Villani, Krishnan K. Mahadevan, Matteo Ligorio—shared first authorship. Vikram Deshpande and Cristina R. Ferrone—shared last authorship.
Rights and permissions
About this article
Cite this article
Villani, V., Mahadevan, K.K., Ligorio, M. et al. Phosphorylated Histone H3 (PHH3) Is a Superior Proliferation Marker for Prognosis of Pancreatic Neuroendocrine Tumors. Ann Surg Oncol 23 (Suppl 5), 609–617 (2016). https://doi.org/10.1245/s10434-016-5171-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5171-x