Abstract
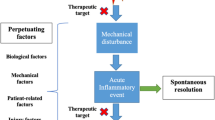
Rheumatoid arthritis (RA) and osteoarthritis are chronic diseases that result in cartilage degradation and loss of joint function. Currently available drugs are predominantly directed towards the control of pain and/or the inflammation associated with joint synovitis but they do little to reduce joint destruction. In the future, it will be important to have drugs that prevent the structural damage caused by bone and cartilage breakdown.
In this review, we will outline the structure and function of cartilage and the key features of matrix metalloproteinases (MMPs), enzymes involved in joint destruction. We will present evidence for the role of MMPs in RA and osteoarthritis, and describe the potential of synthetic inhibitors to control MMP activity and so prevent joint destruction.
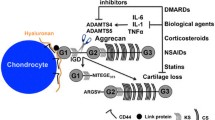
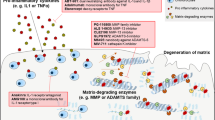
MMPs are able to cleave all components of the cartilage matrix. Regulation of MMPs is aberrant in osteoarthritis and RA, and MMPs have been implicated in the collagen breakdown that contributes to joint destruction in these diseases. Synthetic MMP inhibitors have been developed. In animal models of osteoarthritis and/or RA, these agents have shown chondroprotective effects. However, results from clinical trials in RA have been equivocal, with some studies being terminated because of lack of efficacy or safety concerns. Nevertheless, this approach remains promising. Increased understanding of the structure, regulation and function of individual MMPs may lead to more effective strategies, and approaches aimed at multiple steps of the pathogenesis of arthritis may be needed to break the chronic cycle of joint destruction.










Similar content being viewed by others
References
Conaghan PG, Brooks P. Disease-modifying antirheumatic drugs, including methotrexate, gold, antimalarials, and D-penicillamine. Curr Opin Rheumatol 1995; 7(3): 167–73
Hardingham TE, Fosang AJ. Proteoglycans: many forms and any functions. FASEB J 1992; 6: 861–70
Muir H. The chondrocyte, architect of cartilage: biomechanics, structure, function and molecular biology of cartilage matrix macromolecules. Bioessays 1995; 17: 1039–48
Dingle JT, Page TDP, King B, et al. In vivo studies of articular tissue damage mediated by catabolin/interleukin 1. Ann Rheum Dis 1987; 46: 527–33
Page-Thomas DP, King B, Stephens T, et al. In vivo studies of cartilage regeneration after damage induced by catabolin/interleukin-1. Ann Rheum Dis 1991; 50: 75–80
Fell HB, Barratt ME, Welland H, et al. The capacity of pig articular cartilage in organ culture to regenerate after breakdown induced by complement-sufficient antiserum to pig erythrocytes. Calcif Tissue Res 1976; 20(1): 3–21
van der Rest M, Garrone R. Collagen family of proteins. FASEB J 1991; 5(13): 2814–23
Jubb RW, Fell HB. The breakdown of collagen by chondrocytes. J Pathol 1980; 130: 159–62
Cawston TE. Proteinases and inhibitors. Br Med Bull 1995; 51: 385–401
Birkedal-Hansen H, Moore WGI, Bodden MK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 1993; 4: 197–250
Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A 1990; 87: 5578–82
Henriet P, Blavier L, DeClerck YA. Tissue inhibitors of metalloproteinases (TIMP) in invasion and proliferation. APMIS 1999; 107: 111–9
Collier IE, Wilhelm SM, Eisen AZ, et al. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem 1988; 263(14): 6579–87
Wilhelm SM, Collier IE, Marmer BL, et al. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem 1989; 264(29): 17213–21
Allan JA, Docherty AJP, Barker PJ, et al. Binding of gelatinases A and B to type-I collagen and other matrix components. Biochem J 1995; 309: 299–306
Sato H, Takino T, Okada Y, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994; 370: 61–5
Sato H, Kinoshita T, Takino T, et al. Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TTMP)-2. FEBS Lett 1996; 393(1): 101–4
Pei D, Weiss SJ. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature 1995; 375(6528): 244–7
Matrisian LM. The matrix-degrading metalloproteinases. Bioessays 1992; 14: 455–63
Clark IM, Rowan A, Cawston TE. Matrix metalloproteinase inhibitors in the treatment of arthritis. Curr Opin Anti-inflamm Immunomod Invest Drugs 2000; 2(1): 16–25
Shingleton WD, Hodges DJ, Brick P, et al. Collagenase: a key enzyme in collagen turnover. Biochem Cell Biol 1996; 74(6): 759–75
Welgus HG, Jeffrey JJ, Stricklin GP, et al. Characteristics of the action of human skin fibroblast collagenase on fibrillar collagen. J Biol Chem 1980; 255(14): 6806–13
Hasty KA, Jeffrey JJ, Hibbs MS, et al. The collagen substrate specificity of human neutrophil collagenase. J Biol Chem 1987; 262(21): 10048–52
Knauper V, Lopez-Otin C, Smith B, et al. Biochemical characterisation of human collagenase-3. J Biol Chem 1996; 271: 1544–50
Cawston TE, Rowan AD. Prevention of cartilage breakdown by matrix metalloproteinase inhibition: a realistic therapeutic target? Br J Rheumatol 1998; 37: 353–6
Clark IM, Powell LK, Ramsey S, et al. The measurement of collagenase, tissue inhibitor of metalloproteinases (TIMP), and collagenase-TIMP complex in synovial fluids from patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum 1993; 36: 372–9
Mort JS, Dodge GR, Roughley PJ, et al. Direct evidence for active metalloproteinases mediating matrix degradation in interleukin 1-stimulated human articular cartilage. Matrix 1993; 13(2): 95–102
Dodge GR, Pidoux I, Poole AR. The degradation of type II collagen in rheumatoid arthritis: an immunoelectron microscopic study. Matrix 1991; 11(5): 330–8
McCachren SS. Expression of metalloproteinases and metalloproteinase inhibitor in human arthritic synovium. Arthritis Rheum 1991; 34(9): 1085–93
Brinckerhoff CE. Joint destruction in arthritis: metalloproteinases in the spotlight. Arthritis Rheum 1991; 34(9): 1073–5
Arner EC, Pratta MA, Trzaskos JM, et al. Generation and characterization of aggrecanase — a soluble, cartilage-derived aggrecan-degrading activity. J Biol Chem 1999; 274(10): 6594–601
Abbaszade I, Liu RQ, Yang F, et al. Cloning and characterization of ADAMTS11, an aggrecanase from the ADAMTS family. J Biol Chem 1999; 274(33): 23443–50
Billington CJ, Clark IM, Cawston TE. An aggrecan-degrading activity associated with chondrocyte membranes. Biochem J 1998; 336(1): 207–12
Amour A, Slocombe PM, Webster A, et al. TNF-α converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett 1998; 435(1): 39–44
Brennan FM, Browne KA, Green PA, et al. Reduction of serum matrix metalloproteinase 1 and matrix metalloproteinase 3 in rheumatoid arthritis patients following anti-tumour necrosis factor-a (cA2) therapy. Br J Rheumatol 1997; 36(6): 643–50
Lipsky P, St. Clair W, Furst D, et al. 54 week clinical and radiographic results from the ATTRACT trial: a phase III study of infliximab (Remicade™) in patients with active RA despite methotrexate [abstract]. Arthritis Rheum 1999; 42Suppl. 11: S401
Elliott S, Rowan A, Carrère S, et al. Esculetin inhibits cartilage resorption induced by IL-1 α in combination with OSM. Ann Rheum Dis 2001; 60: 158–65
Martel-Pelletier J, Mineau F, Tardif G, et al. Tenidap reduces the level of interleukin 1 receptors and collagenase expression in human arthritic synovial fibroblasts. J Rheumatol 1996; 23: 24–31
Beckett RP, Davidson AH, Drummond AH, et al. Recent advances in matrix metalloproteinase inhibitor research. Drug Disc Today 1995; 1: 16–26
Bottomley KM, Johnson WH, Walter DS. Matrix metalloproteinase inhibitors in arthritis. J Enzyme Inhib 1998; 13(2): 79–101
Martel-Pelletier, Pelletier JP. Wanted: the collagenase responsible for the destruction of the collage network in human cartilage. Br J Rheumatol 1996; 35(9): 818–20
Nemunaitis J, Poole C, Primrose J, et al. Combined analysis of studies of the effects of the matrix metalloproteinase inhibitor marimastat on serum tumor markers in advanced cancer: selection of a biologically active and tolerable dose for longer-term studies. Clin Cancer Res 1998; 4(5): 1101–9
Shapiro SD. Mighty mice: transgenic technology ‘knocks out’ questions of metalloproteinase function. Matrix Biol 1997; 15: 527–33
Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 1997; 385(6618): 729–33
Konttinen YT, Ceponis A, Takagi M, et al. New collagenolytic enzymes cascade identified at the pannus-hard tissue junction in rheumatoid arthritis: destruction from above. Matrix Biol 1998; 17(8–9): 585–601
Knauper V, Will H, Lopez-Otin C, et al. Cellular mechanisms for human procollagenase-3 (MMP-13) activation: evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem 1996; 271(29): 17124–31
Brewster M, Lewis EJ, Wilson KL, et al. Ro 32-3555, an orally active collagenase selective inhibitor, prevents structural damage in the STR/ORT mouse model of osteoarthritis. Arthritis Rheum 1998; 41(9): 1639–44
Greenwald RA. Treatment of destructive arthritic disorders with MMP inhibitors: potential role of tetracyclines. Ann NY Acad Sci 1994; 732: 181–98
Greenwald RA, Golub LM, Ramamurthy NS, et al. In vitro sensitivity of the three mammalian collagenases to tetracycline inhibition: relationship to bone and cartilage degradation. Bone 1998; 22(1): 33–8
Cawston TE. Metalloproteinase inhibitors and the prevention of connective tissue breakdown. Pharmacol Ther 1996; 70(3): 163–82
Shaw, T. Inhibition of matrix metalloproteinases in rheumatoid arthritis. In: Firestein GS, Panayi GS, Wollheim F, editors. Rheumatoid arthritis. Oxford: Oxford University Press, 2000: 551–61
Butler GS, Hutton M, Wattam BA, et al. The specificity of TIMP-2 for matrix metalloproteinases can be modified by single amino acid mutations. J Biol Chem 1992; 274(29): 20391–6
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Elliott, S., Cawston, T. The Clinical Potential of Matrix Metalloproteinase Inhibitors in the Rheumatic Disorders. Drugs & Aging 18, 87–99 (2001). https://doi.org/10.2165/00002512-200118020-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002512-200118020-00002