Summary
Tacrolimus, a novel macrocyclic lactone with potent immunosuppressive properties, is currently available as an intravenous formulation and as a capsule for oral use, although other formulations are under investigation.
Tacrolimus concentrations in biological fluids have been measured using a number of methods, which are reviewed and compared in the present article. The development of a simple, specific and sensitive assay method for measuring concentrations of tacrolimus is limited by the low absorptivity of the drug, low plasma and blood concentrations, and the presence of metabolites and other drugs which may interfere with the determination of tacrolimus concentrations. Currently, most of the pharmacokinetic data available for tacrolimus are based on an enzyme-linked immunosorbent assay method, which does not distinguish tacrolimus from its metabolites.
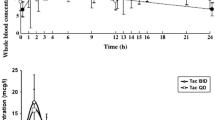
The rate of absorption of tacrolimus is variable with peak blood or plasma concentrations being reached in 0.5 to 6 hours; approximately 25% of the oral dose is bioavailable. Tacrolimus is extensively bound to red blood cells, with a mean blood to plasma ratio of about 15; albumin and α1-acid glycoprotein appear to primarily bind tacrolimus in plasma. Tacrolimus is completely metabolised prior to elimination. The mean disposition half-life is 12 hours and the total body clearance based on blood concentration is approximately 0.06 L/h/kg. The elimination of tacrolimus is decreased in the presence of liver impairment and in the presence of several drugs.
Various factors that contribute to the large inter- and interindividual variability in the pharmacokinetics of tacrolimus are reviewed here. Because of this variability, the narrow therapeutic index of tacrolimus, and the potential for several drug interactions, monitoring of tacrolimus blood concentrations is useful for optimisation of therapy and dosage regimen design.
Similar content being viewed by others
References
Zeevi A, Duquesnoy R, Eiras G, et al. Immunosuppressive effect of FK506 on in vitro lymphocyte alloactivation. Transplant Proc 1987; 19: 40–4
Starzl TE, Todo S, Fung J, et al. FK 506 for liver, kidney and pancreas transplantation. Lancet 1989; ii: 1000–4
Todo S, Fung J, Starzl TE, et al. Liver, kidney and thoracic organ transplantation under FK506. Ann Surg 1990; 212: 295–302
Honbo T, Kobayashi M, Hane K, et al. The oral dosage form of FK-506. Transplant Proc 1987; 19Suppl. 6: 17–22
Taormino D, Abdallah HY, Venkataramanan R, et al. Stability and sorption of FK 506 in 5% dextrose injection and 0.9% sodium chloride injection in glass, polyvinyl chloride, and polyolefin containers. Am J Hosp Pharm 1992; 49(1): 119–22
Croyle MA, Venkataramanan R, Burckart GJ, et al. Adsorption of pediatric intravenous doses of tacrolimus in 5% dextrose to IV administration sets. Am J Health-Syst Pharm 1995. In press
Ko S, Nakajima Y, Kanehiro H, et al. The pharmacokinetic benefits of newly developed liposome-incorporated FK506. Transplantation 1994; 58: 1142–4
Ko S, Nakajima Y, Kanehiro H, et al. Significance of newly developed liposomal FK 506 in canine liver transplantation. Transplant Proc 1995; 1: 351–3
Lee M, Straubinger RM, Jusko WJ. Physicochemical, pharmacokinetics and pharmacodynamic evaluation of liposomal tacrolimus (FK506) in rats. Pharm Res 1995; 12: 1055–9
Freeman DJ, Stawecki M, Howson B. Stability of FK506 in whole blood samples. Ther Drug Monit 1995; 17: 266–7
Ingels SC, Koenig J, Scott MG. Stability of FK506 (Tacrolimus) in whole blood specimens. Clin Chem 1995; 41: 1320–1
Tamura K, Kobayashi M, Hashimoto K, et al. A highly sensitive method to assay FK506 levels in plasma. Transplant Proc 1987; 19Suppl. 6: 23–9
Cadoff E, Venkataramanan R, Krajack A, et al. Assay of FK 506 in plasma. Transplant Proc 1990; 22: 50–1
Warty VS, Venkataramanan R, Zendehrough P, et al. Practical aspects of FK506 analysis (Pittsburgh Experience). Transplant Proc 1991; 23: 2730–1
Wallemacq PE, Firdaous I, Hassoun A. Improvement and assessment of enzyme-linked immunosorbent assay to detect low FK506 concentration in plasma or whole blood within 6 hours. Clin Chem 1993; 39: 1045–9
Kobayashi M, Tamura K, Katayama N, et al. FK 506 assay past and present — characteristics of FK 506 ELISA. Transplant Proc 1991; 23: 2725–9
Jusko WJ, D’Ambrosio R. Monitoring FK506 concentrations in plasma and whole blood. Transplant Proc 1991; 23: 2732–5
D’Ambrosio R, Girzaitis N, Jusko WJ. Validation and quality assurance program for monitoring tacrolimus (FK 506) concentrations in plasma and whole blood. Ther Drug Monit 1993; 15: 414–26
Warty V, Zuckerman S, Venkataramanan R, et al. Tacrolimus analysis: a comparison of different methods and matrices. Ther Drug Monit 1995; 17: 159–67
Winkler M, Christians U, Stoll K, et al. Comparison of different assays for the quantitation of FK 506 levels in blood or plasma. Ther Drug Monit 1994; 16: 281–6
D’Ambrosio R, Girzaitis N, Jusko WJ. Multicenter comparison of tacrolimus (FK 506) whole blood concentrations as measured by the Abbott IMx analyzer and enzyme immunoassay with methylene chloride extraction. Ther Drug Monit 1994; 16: 287–92
Grenier FC, Luczkiw T, Bergmann M, et al. A whole blood FK506 assay for the IMx analyzer. Transplant Proc 1991; 23: 2748–9
Matsunami H, Tada A, Makuchi M, et al. New technique for measuring tacrolimus concentrations in blood [letter]. Am J Hosp Pharm 1994; 51: 123
Friob MC, Hassoun A, Latinne D, et al. A combined HPLCELISA evaluation of FK506 in transplant patients. Transplant Proc 1991; 23: 2750–2
Warty VS, Zuckerman S, Venkataramanan R, et al. FK506 measurement: comparison of different analytical methods. Ther Drug Monit 1993; 15: 204–8
Takada K, Oh-Hashi M, Yoshikawa H, et al. Determination of a novel potent immunosuppressant (FK506) in rat serum and lymph by high-performance liquid chromatography with chemiluminescence defection. J Chromatogr 1990; 530: 212–8
Christians U, Braun F, Schmidt M, et al. Specific and sensitive measurement of FK506 and its metabolites in blood and urine of liver-graft recipients. Clin Chem 1992; 38: 2025–32
Zeevi A, Eiras G, Burckart G, et al. Bioassay of plasma specimens from liver transplant patients on FK506 immunosuppression. Transplant Proc 1990; 22Suppl. 1: 60–3
Murthy JN, Chen Y, Warty VS, et al. Radioreceptor assay for quantifying FK506 immunosuppressant in whole blood. Clin Chem 1992; 7: 1307–10
Winkler M, Christians U, Gonschior AK, et al. Evaluation of the new Pro-Trac Tacrolimus monoclonal whole blood ELISA for monitoring Tacrolimus levels in patients after kidney, heart and liver transplant [abstract]. Ther Drug Monit 1995; 17: 430
Beysens AJ, Beuman GH, Van der Heijden JJ, et al. Determination of tacrolimus (FK506) in whole blood using liquid chromatography and fluorescent detection. Chromatographia 1994; 39: 490–6
Wallemacq PE, Reding R. FK506 (Tacrolimus), a novel immunosuppressant in organ transplantation: clinical, biomedical and analytical aspects. Clin Chem 1993; 39: 2219–27
Venkataramanan R, Jain A, Lever J, et al. High dose tacrolimus requirement in transplant patients [abstract]. Pharm Res 1994; 11: S405
Winkler M, Ringe B, Rodeck B, et al. The use of plasma levels for FK506 dosing in liver grafted patients. Transplant Int 1994; 7: 329–33
Beysens AJ, Wijnen RMH, Beuman GH, et al. FK 506: monitoring in plasma or in whole blood? Transplant Proc 1991; 23: 2745–7
Firdaous I, Hassoun A, Otte JB, et al. Development of an experimental specific assay to determine tacrolimus whole blood levels in hepatic and renal transplant patients. Clin Chem 1995; 41: 1292–6
Christians U, Kruse C, Kownatzki R, et al. Measurement of FK 506 by HPLC and isolation and characterization of its metabolites. Transplant Proc 1991; 23: 940–1
Christians U, Braun F, Kosian N, et al. High-performance liquid chromatography mass spectrometry of FK506 and its metabolites in blood, bile, and urine of liver grafted patients. Transplant Proc 1991; 23: 2741–4
Gonschior AK, Christians U, Winkler M, et al. Simplified high performance liquid chromatography-mass spectrometry assay for measurement of Tacrolimus and its metabolites and its cross validation with microparticulate enzyme immunoassay. Ther Drug Monit 1995. In Press
Zeevi A, Venkataramanan R, Warty V, et al. In vitro assessment of FK 506 immunosuppressive activity in transplant patients. Transplant Proc 1991; 23: 2897–9
Zeevi A, Eiras G, Kaufman C, et al. Correlation between bioassayed plasma levels of FK506 and lymphocyte growth from liver transplant biopsies with histological evidence of rejection. Transplant Proc 1991; 23: 1406–8
Gruber SA, Hewitt JM, Sorenson AL, et al. Pharmacokinetics of FK506 after intravenous and oral administration in patients awaiting renal transplantation. J Clin Pharmacol 1994; 34: 859–64
Venkataramanan R, Jain A, Warty VS, et al. Pharmacokinetics of FK506 following oral administration: a comparison of FK 506 and cyclosporine. Transplant Proc 1991; 23: 931–3
Venkataramanan R, Jain A, Warty VS, et al. Pharmacokinetics of FK 506 in transplant patients. Transplant Proc 1991; 23: 2736–40
Venkataramanan R, Jain A, Abu-Elmagd K, et al. Pharmacokinetics of FK506 in liver transplant patients [abstract]. Pharm Res 1991; 8: S313
Peters DH, Fitton A, Plosker GL, et al. Tacrolimus: a review of its pharmacology, and therapeutic potential in hepatic and renal transplantation. Drugs 1993; 46: 746–94
Hooks MA. Tacrolimus, a new immunosuppressant — a review of the literature. Ann Pharmacother 1994; 28: 501–10
Steinmüller DR. FK506 and organ transplantation. Austin (TX): Medical Intelligence Unit, RG Landers Co., 1994; 1–111
Venkataramanan R, Warty VS. Pharmacokinetics and monitoring of FK506 [Tacrolimus]. In: Thompson AW, Starzl TE, editors. Immunosuppressive drugs. London: Arnold Publishers, 1994: 80–91
Kelly PA, Burckart GJ, Venkataramanan R. Tacrolimus: a new immunosuppressive agent. Am J Health-Syst Pharm 1995; 52: 1521–35
Jusko WJ, Piekoszewski W, Klintmalm GB, et al. Pharmacokinetics of tacrolimus in liver transplant patients. Clin Pharmacol Ther 1995; 57: 281–90
Böttiger Y, Rafael G, Brattsröm C, et al. Tacrolimus (FK506) kinetics in liver and kidney transplant recipients [abstract]. Ther Drug Monit 1995; 17: 416
Swaminathan A, Burckart G, Venkataramanan R. Uptake of FK506 by rat intestinal rings [abstract]. Pharm Res 1993; 10: S389
Lee C, Hewitt J, Aweeka F, et al. Pharmacokinetics of tacrolimus (FK506) prior to kidney transplantation [abstract]. Clin Pharmacol Ther 1994; 53: 151
Aweeka FT, Benet LZ, Gambertoglio JG, et al. Comparative pharmacokinetics of orally (PO) and intravenously (IV) administered tacrolimus (FK506) in pre- and post-kidney transplant recipients [abstract]. Clin Pharmacol Ther 1993 Feb; 53: 151
Yasuhara M, Hashida T, Toraguchi M, et al. Pharmacokinetics and pharmacodynamics of FK506 in pediatric patients receiving living-related donor liver transplantations. Transplant Proc 1995; 27: 1108–10
Jain AB, Venkataramanan R, Todo S, et al. Intravenous, oral pharmacokinetics, and oral dosing of FK 506 in small bowel transplant patients. Transplant Proc 1992; 24: 1181–2
Iwasaki K, Shiraga T, Nagase K, et al. Pharmacokinetic study of FK506 in the rat. Transplant Proc 1991; 23: 2757–9
Jain AB, Venkataramanan R, Cadoff E, et al. Effect of hepatic dysfunction on FK 506 pharmacokinetics and trough concentrations. Transplant Proc 1990; 22Suppl. 1: 57–9
Furukawa H, Imventarza O, Venkataramanan R, et al. The effect of bile duct ligation and bile diversion on FK506 pharmacokinetics in dogs. Transplantation 1992; 53: 722–5
Ericzon B-G, Ekqvist B, Groth CG, et al. Pharmacokinetics of FK506 during maintenance in liver transplant patients. Transplant Proc 1991; 23: 2775–6
Machida M, Takahara S, Ishibashi M, et al. Effect of temperature and hematocrit on plasma concentration of FK506. Transplant Proc 1991; 23: 2753–4
Habucky K. FK506: its pharmacokinetics and interactions with other drugs [dissertation]. Pittsburgh: Univ. of Pittsburgh. 1992
Kay JE, Kwateng-SE, Geraghty F, et al. Uptake of FK506 by lymphocytes and erythrocytes. Transplant Proc 1991; 23: 2760–2
Habucky K, Flowers J, Warty VS, et al. Blood protein binding (BPB) of FK506 (F) in various species [abstract]. Pharm Res 1992; 9 Suppl.: 334
Piekoszewski W, Jusko WJ. Plasma protein binding of tacrolimus in humans. J Pharm Sci 1993; 82: 340–1
Nagase K, Iwasaki K, Nozaki K, et al. Distribution and protein binding of FK506, a potent immunosuppressive macrolide lactone in human blood and its uptake by erythrocytes. J Pharm Pharmacol 1994; 46: 113–7
Warty V, Venkataramanan R, Zendehrough P, et al. Distribution of FK506 in plasma lipoproteins. Transplant Proc 1991; 23: 954–5
Piekoszewski W, Chow FS, Jusko WJ. Disposition of tacrolimus (FK506) in rabbits. Drug Metab Dispos 1993; 21: 690–8
Sanghvi A, Warty VS, Diven WF, et al. Increased cyclosporine uptake by cells pre treated with FK506 and evidence for binding to a common intracellular protein. Transplant Proc 1989; 21: 1050–2
Warty V, Diven W, Cadoff E, et al. FK506: a novel immunosuppressive agent: characteristics of binding and uptake by human lymphocytes. Transplantation 1988; 46: 453–5
Venkataramanan R, Jain A, Cadoff E, et al. Pharmacokinetics of FK506: preclinical and clinical studies. Transplant Proc 1990; 22: 52–6
Wijnen RMH, Ericzon BG, Tiebosch ATGM, et al. Toxicity of FK506 in the cynomolgus monkey: non correlation with FK506 serum levels. Tranplant Proc 1991; 23: 3101–4
Jain A, Venkataramanan R, Fung J, et al. Pregnancy in liver transplant patients under FK506 regimen. Transplantation 1993; 53: 1588–9
Christians U, Radeke HH, Kownatzki R, et al. Isolation of immunosuppressive metabolite of FK506 generated by human microsome preparations. Clin Biochem 1991; 24: 271–5
Lhoest G, Wallemacq P, Verbeeck R. Isolation and mass spec-trometric identification of five metabolites of FK506, a novel macrolide immunosuppressive agent, from human plasma. Pharm Acta Helv 1991; 66: 302–6
Lhoest G, Maton N, Verbeeck R. Isolation and mass spectrometric identification of two metabolites of FK 506 from rat liver microsomal incubation media. Pharm Acta Helv 1992; 67: 270–4
Lhoest G, Maton N, Verbeeck R. Isolation and identification of a novel isomerized epoxide metabolite of FK-506 from eryth-romycin-induced rabbit liver microsomes. Drug Metab Dispos 1993; 21: 850–4
Lhoest G, Maton N, Latinne D, et al. 15-Desmethyl FK-506 and 15,31-desmethyl FK-506 from human liver microsomes: isolation, identification (by fast atom bombardment mass spectrometry and nmr), and evaluation of in vitro immunosuppressive activity. Clin Chem 1994; 40: 740–4
Lhoest G, Maton N, Laurent A, et al. Isolation and identification of a FK-506 C36-C37 dihydrodiol from erythromycin-induced rabbit liver microsomes. J Pharm Biomed Anal 1994; 12: 235–41
Sattler M, Guengerich FP, Yun C-Ho, et al. Cytochrome P4503A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab Dispos 1992; 20: 753–61
Venkataramanan R, Warty VS, Zuckerman S, et al. Metabolism of FK506 in transplant patients [abstract]. Pharm Res 1992; 10: 300
Vincent SH, Karanam BV, Painter SK et al. In vitro metabolism of FK-506 in rat, rabbit, and human liver microsomes: identification of a major metabolite and of cytochrome P450 3A as the major enzymes responsible for its metabolism. Arch Biochem Biophys 1992; 294: 454–60
Iwasaki K, Matsuda H, Nagase K, et al. Effects of twenty-three drugs on the metabolism of FK506 by human liver microsomes. Res Commun Chem Path Pharmacol 1993; 82: 209–17
Iwasaki K, Shiraga T, Nagase K, et al. Isolation, identification, and biological activities of oxidative metabolites of FK506, a potent immunosuppressive macrolide lactone. Drug Metab Dispos 1993; 21: 971–7
Iwasaki K, Shiraga T, Matsuda H, et al. Identification and biological activities of the metabolites oxidized at multiple sites of FK506. Drug Metab Dispos 1995; 23: 28–34
Karanam BV, Vincent SH, Lee Chiu SH. FK 506 metabolism in human liver microsomes: investigation of the involvement of cytochrome P450 isozymes other than CYP3A4. Drug Metab Dispos 1994; 22: 811–4
Perotti TBY, Prueksaritanont T, Benet LZ, et al. HPLC assay for FK 506 and two metabolites in isolated rat hepatocytes and rat liver microsomes. Pharm Res 1994; 6: 844–7
Perotti TBY, Okudaira N, Prueksaitanont T, et al. FK 506 metabolism in male and female rat liver microsomes. Drug Metab Dispos 1994; 23: 85–91
Gusev AL, Muddiman DC, Proctor A, et al. In vitro hepatic metabolism of tacrolimus (FK506) using SIMS and MALDI mass spectrometry [abstract]. 43rd ASMS Conference on Mass Spectrometry and Allied Topics: 1995 May 21–26; Atlanta (GA), 560
Tata P, Venkataramanan R, Subbotina N, et al. Species dependent hepatic metabolism of tacrolimus (FK506) [abstract]. Pharm Res 1994; 11: S408
Ahmed SS, Strobel HW, Napoli KL et al. Adrenochrome reaction implicates oxygen radicals in metabolism of cyclosporin A and FK506 in rat and human liver microsomes. J Pharmacol Exp Ther 1993; 265: 1047–54
Stiff D, Venkataramanan R, Prasad T. Metabolism of FK 506 in differentially induced rat liver microsomes. Res Commun Chem Pathol Pharmacol 1992; 78: 121–4
Prasad T, Stiff D, Subbotina N, et al. FK 506 (tacrolimus) metabolism by rat liver microsomes and its inhibition by other drugs. Res Commun Chem Path Pharmacol 1994; 84: 35–46
Gonschior AK, Christians U, Winkler M, et al. Tacrolimus (FK506) metabolite pattern in blood from liver and kidney transplant patients [abstract]. Ther Drug Monit 1995; 17: 417
Gonschior AK, Christians U, Braun F, et al. Measurement of blood concentrations of FK506 (tacrolimus) and its metabolites in liver graft patients after the first dose by hplc-ms and microparticulate enzyme immunoassay (MEIA). Br J Clin Pharmacol 1994; 38: 567–1
Jain AB, Abu-Elmagd K, Abdallah H, et al. Pharmacokinetics of FK506 in liver transplant recipients after continuous intravenous infusion. J Clin Pharmacol 1993; 33: 606–11
Venkataramanan R, Warty V, Zemaitis MA, et al. Biopharmaceutical aspects of FK506. Transplant Proc 1987; 19: 30–5
Jain AB, Fung JJ, Tzakis AG, et al. Comparative study of cyclosporine and FK 506 dosage requirements in adult and pediatric orthotopic liver transplant patients. Transplant Proc 1991; 23: 2763–6
Mekki Q, Lee C, Carrier S, et al. The effect of food on oral bioavailability of tacrolimus (FK506) in liver transplant patients [abstract]. Clin Pharmacol Ther 1994; 53: 229
Aweeka FT, Benet LZ, Gambertoglio JG, et al. Comparative pharmacokinetics of orally (PO) and intravenously (IV) administered tacrolimus (FK506) in pre- and post-kidney transplant recipients. Clin Pharmacol Ther 1994; 2: 151
Uchida K and the Japanese FK506 Study Group. Japanese study of FK506 on kidney transplantation: the benefit of monitoring the whole blood FK506 concentrations in patients. Transplant Proc 1991; 23: 3085–8
Gravel J, Marshall G, Kerman RA. Pilot study of reversibility of immunosuppression and pharmacokinetics of a single iv dose of FK506 in healthy male volunteers [internal report]. Deerfield (IL): Fujisawa Pharmaceuticals USA Inc., 1991
Jusko WJ, Thompson A, Fung J, et al. Consensus document: therapeutic monitoring of Tacrolimus (FK506). Ther Drug Monit 1995. In press
McDiarmid SV, Colonna II JO, Shaked A, et al. Differences in oral FK506 dose requirements between adult and pediatric liver transplant patients. Transplantation 1993; 55: 1328–32
Steeves M, Abdallah HY, Venkataramanan R, et al. In-vitro interaction of a novel immunosuppressant, FK506, and antacids. J Pharm Pharmacol 1991; 43: 574–7
Sewing K-Fr. Pharmacokinetics, dosing principles, and blood level monitoring of FK506. Transplant Proc 1994; 26: 3267–9
Jensen C, Jordan M, Shapiro R, et al. Interaction between tacrolimus and erythromycin [letter]. Lancet 1994; 344: 825
Mieles L, Venkataramanan R, Yokoyama I, et al. Interaction between FK506 and clotrimazole in a liver transplant recipient. Transplantation 1992; 52: 1086–7
Manez R, Martin M, Raman D, et al. Fluconazole therapy in transplant recipients receiving FK506. Transplantation 1994; 57: 1521–35
Shapiro R, Venkataramanan R, Warty VS, et al. FK 506 interaction with danazol. Lancet 1993; 341: 1344–5
Rui X, Flowers J, Warty V, et al. Drug interactions with FK 506 [abstract]. Pharm Res 1992; 9: S291
Jain AB. Venkataramanan R, Fung J, et al. Pharmacokinetics of cyclosporine and nephrotoxicity in orthotopic liver transplant patients rescued with FK 506. Transplant Proc 1991; 23: 2777–9
Wu YM, Venkataramanan R, Suzuki M, et al. Interaction between FK 506 and cyclosporine in dogs. Transplant Proc 1991; 23: 2797–9
Christians U, Braun F, Sattler M, et al. Interactions of FK506 and cyclosporine metabolism. Transplant Proc 1991; 23: 2794–6
Farghali H, Sakr M, Gasbarrini A, et al. Effect of FK506 chronic administration on bromosulpthalein hepatic excretion in rats. Transplant Proc 1991; 23: 2802–4
Moochhala SM, Lee EJD, Earnest L, et al. Inhibition of drug metabolism in rat and human liver microsomes by FK506 and cyclosporine. Tranplant Proc 1991; 23: 2786–8
Omar G, AD, Lhoest G, Ottson et al. FK506 inhibition of cyclosporine metabolism by human liver microsomes. Transplant Proc 1991; 23: 934–5
Pichard L, Fabre I, Domergue J, et al. Effect of FK506 on human hepatic cytochromes P-450: interaction with CyA. Transplant Proc 1991; 23: 2791–3
Shah IA, Whiting PH, Omar G, et al. FK 506 metabolism and drug interactions: effects of FK 506 on human hepatic microsomal cytochrome P-450-dependent drug metabolism in vitro. Transplant Proc 1991; 23: 2783–5
Vincent SH, Wang RW, Karanam BV, et al. Effects of the immunosuppressant FK-506 and its analog FK-520 on hepatic and renal cytochrome P450 mixed-function oxidase. Biochem Pharmacol 1991; 293: 1325–30
Habucky K, Venkataramanan R, Rui Xiashon, et al. The pharmacodynamics of pentobarbital following FK506 therapy. Transplant Proc 1991; 23: 2800–1
Hopp L, Lombardozzi S, Gilboa N, et al. Removal of FK506 by continuous ultrafiltration in a patient with liver failure: a case report. Clin Transpl 1992; 7: 546–51
Abu-Elmagd KM, Fung JJ, Alessiani M, et al. The effect of graft function on FK506 plasma levels, dosages, and renal function, with particular reference to the liver. Transplantation 1991; 52: 71–7
Abu-Elmagd KM, Fung JJ, Alessiani M, et al. Strategy of FK 506 therapy in liver transplant patients: effect of graft function. Transplant Proc 1991; 23: 2777–80
Alessiani M, Cillo U, Fung JJ, et al. Adverse effects of FK506 overdose after liver transplantation. Transplant Proc 1993; 25: 628–34
Winkler M, Jost U, Ringe B, et al. Association of elevated FK 506 plasma levels with nephrotoxicity in liver-grafted patients. Transplant Proc 1991; 23: 3153–5
Backman L, Nicar M, Levy M, et al. FK506 trough levels in whole blood and plasma in liver transplant recipients. Transplantation 1994; 57: 519–25
Backman L, Levy MF, Klintmalm G and the FK506 Multicenter Study Group. Whole blood and plasma levels of FK506 after liver transplantation: results from the US Multicenter Trial. Transplant Proc 1995; 27: 1124
Winkler M, Ringe B, Jost U, et al. Plasma level guided low dose FK506 therapy in patients with early liver dysfunction after liver transplantation. Transplant Proc 1993; 25: 2688–90
Schwartz M, Hoist B, Fackman D, et al. and the US Multicenter FK506 Dose Optimization Study Group. FK506 in liver transplantation: correlation of whole blood levels with efficacy and toxicity. Transplant Proc 1995; 27: 1107
Jain AB, Todo S, Fung JJ, et al. Correlation of rejection episodes with FK 506 dosage, FK 506 levels following primary orthotopic liver transplant. Transplant Proc 1991; 23: 3023–5
Japanese FK 506 Study Group. Japanese study of FK 506 on kidney transplantation: the benefit of monitoring the whole blood FK 506 concentrations in patients. Transplant Proc 1991; 23: 3085–8
Sandbom WJ, Lawson GM, Cody TJ, et al. Early cellular rejection after orthotopic liver transplantation correlates with low concentrations of FK506 in hepatic tissue. Hepatology 1995; 21: 70–6
Venkataramanan R, Lever J, Jain A, et al. FK506: correlation between trough concentrations and aucs in transplant patients [abstract]. Pharm Res 1993; 10: S390
Chou D. Therapeutic drug monitoring (immunosuppressive drugs). Clin Chem 1993; 12: 412–5
Ptachcinski R, Burckart G, Venkataramanan R. Cyclosporine concentration determinations for monitoring and pharmacokinetic studies. J Clin Pharmacol 1986; 26: 358–66
Shaefer MS, Collier DS, Haven MC, et al. Falsely elevated FK-506 levels caused by sampling through central venous catheters. Transplantation 1993; 56: 475–6
Jain A, Pinna A, Fung J, et al. Capillary blood versus arterial or venous blood for tacrolimus monitering in clinical transplantation. Transplantation 1995; 60: 512–4
McMichael J, Irish W, McCauley G, et al. Evaluation of a novel ‘intelligent’ dosing system for optimizing FK506 therapy. Transplant Proc 1991; 23: 2780–2
Lieberman R. FK506, artificial intelligence and phar-macoeconomics. J Clin Pharmacol 1993; 33: 596–8
McMichael J, Lieberman R, Irish W, et al. Three dimensional surface mapping and simulation of FK506 dose — level relationships [abstract]. In: Anderson, Katzper, editors. Proceedings of the 1993 Western Simulation Multiconference, Simulation in Health Sciences and Services: 1994 Jan 24–26; Tempe (AZ), 35–41
McMichael J, Lieberman R, Doyle H, et al. An intelligent and cost-effective computer dosing system for individualizing FK506 dosing in transplantation and autoimmune disorders. J Clin Pharmacol 1993; 33: 599–605
Steinmüller DR, Fitzsimmons WE, Self SG. Model for prediction of FK506 dosing [abstract]. Clin Pharmacol Ther 1994; 53: 181
Cakaloglu Y, Tredger M, Delvin J, et al. Importance of cytochrome P-450IIIA activity in determining dosage and blood levels of FK 506 and cyclosporine in liver transplant recipients. Hepatology 1994; 20: 309–16
Starzl TE, Abu-Elmagd K, Tzakis S, et al. Selected topics on FK506, with special reference to rescue of extrahepatic whole organ grafts, transplantation of “forbidden organs”, side effects, mechanisms and practical pharmacokinetics. Transplant Proc 1991; 23: 914–9
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Venkataramanan, R., Swaminathan, A., Prasad, T. et al. Clinical Pharmacokinetics of Tacrolimus. Clin. Pharmacokinet. 29, 404–430 (1995). https://doi.org/10.2165/00003088-199529060-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-199529060-00003