Summary
Abstract
Cidofovir is an antiviral nucleotide analogue with significant activity against cytomegalovirus (CMV) and other herpesviruses. The drug is indicated for the treatment of CMV retinitis, a sight-threatening condition, in patients with AIDS. Cidofovir has a long intracellular half-life which allows for a prolonged interval (2 weeks) between maintenance doses. In contrast, other intravenous treatment options for patients with CMV retinitis (i.e. ganciclovir and foscarnet) must be administered on a daily basis.
The efficacy of intravenous cidofovir has been demonstrated in patients with AIDS and previously untreated CMV retinitis in multicentre randomised trials, and in a dose-finding study of cidofovir in patients with AIDS and previously treated relapsing CMV retinitis. Clinical trials have been relatively small (n ≤ 100 patients) and no studies have been conducted directly comparing intravenous cidofovir with the more established intravenous agents, ganciclovir or foscarnet. Indirect comparisons of clinical trial data suggest that intravenous cidofovir may have similar efficacy to intravenous ganciclovir or foscarnet in delaying progression of CMV retinitis. However, such comparisons must be made with caution because of potential differences in patient populations, data analysis techniques and interobserver variability in the masked assessment of retinal photographs.
Nevertheless, intravenous cidofovir offers a less intrusive administration regimen than intravenous ganciclovir or foscarnet because of its prolonged dosage interval. Since therapy is life-long, patients receiving daily intravenous ganciclovir or foscarnet (but not cidofovir) usually require an indwelling central venous catheter and are therefore at increased risk of serious infection. The relatively long dosage interval for cidofovir may also have favourable implications in terms of overall treatment costs and patient quality of life, although specificdata are very limited.
Potentially irreversible nephrotoxicity is the major treatment-limiting adverse event associated with intravenous cidofovir in patients with AIDS-related CMV retinitis. Anterior uveitis/iritis has been reported frequently with intravenous cidofovir in postmarketing reports and a small number of patients have developed hypotony. Other treatment options for CMV retinitis are also associated with serious adverse events, and selection of pharmacotherapy will depend on a number of factors including retinitis lesion characteristics, patient quality-of-life issues and efficacy and tolerability profiles of available therapies.
Conclusion: Although the extent of its use may be limited by its adverse event profile, cidofovir offers a useful addition to the limited number of drugs available for the treatment of CMV retinitis in patients with AIDS.
Pharmacodynamic Properties
Cidofovir undergoes activation to its diphosphate metabolite by intracellular kinases which are present in both cytomegalovirus (CMV)-infected and uninfected cells. Cidofovir diphosphate selectively inhibits CMV DNA polymerase and reduces the rate of viral DNA synthesis. Ganciclovir also requires activation by phosphorylation but, unlike cidofovir, requires initial virus-encoded activation by a CMV kinase (an enzyme product of the CMV UL97 gene) which can occur only in CMV-infected cells. In general, in vitro studies have demonstrated that the susceptibility of CMV clinical isolates to cidofovir and the safety margin of cidofovir (selectivity index) are at least comparable to those for ganciclovir. Cidofovir is active against some, but not all, foscarnet-resistant clinical isolates of CMV.
Most ganciclovir-resistant isolates of CMV appear to be UL97 gene product mutants and therefore remain susceptible to cidofovir unless mutations in the DNA polymerase gene are also present. Cross resistance between cidofovir and ganciclovir has been documented in clinical isolates of CMV or strains of CMV selected for resistance in vitro.
There is no ideal animal model of human CMV retinitis because of the species specificity of the virus; however, studies with cidofovir have been conducted in rabbits with experimentally induced herpes simplex virus (HSV)-1 infection of the retina. In this model, pretreatment of eyes with intravitreal injection of cidofovir up to 1 month (or liposome-encapsulated cidofovir up to 8 months) before HSV-1 inoculation prevented or delayed development of retinitis.
Pharmacokinetic Properties
Cidofovir has poor oral bioavailability (<5%) and is therefore administered intravenously. After single dose intravenous administration of cidofovir 3 or 5 mg/kg to individuals with HIV infection (with or without CMV retinitis), peak serum drug concentrations (Cmax) and area under the serum concentration-time curve values (AUC) increased proportionally with dose. Cmax and AUC values were higher when the same cidofovir dose was administered with concomitant oral probenecid (as per cidofovir dosage and administration recommendations) than when administered without probenecid. Binding of cidofovir to plasma proteins is negligible (<7%). Elimination half-life (t½β), renal clearance (CLr) and volume of distribution at steady-state (Vss) were independent of cidofovir dose. Both CLr and Vss were lower when oral probenecid was administered concomitantly, presumably as a result of decreased active tubular secretion of cidofovir. Approximately 90% of an administered dose of cidofovir is eliminated in the urine unchanged. The t½β of cidofovir in serum is approximately 2.5 hours; however, the intracellular half-life of the active metabolite cidofovir diphosphate is ≈65 hours, which may explain the prolonged antiviral effect of the drug.
Therapeutic Use
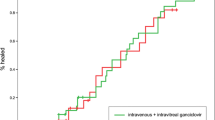
A significantly longer median time to progression of CMV retinitis (primary end-point of studies) was demonstrated with cidofovir (standard regimen) than with deferred treatment (≥120 vs ≈20 days) in patients with AIDS and previously untreated CMV retinitis in 2 small multicentre randomised trials. Patients in the cidofovir group started treatment immediately after randomisation, whereas patients in the deferred treatment groups received treatment only after progression of CMV retinitis occurred.
The standard regimen of cidofovir achieved a significantly longer median time to progression of CMV retinitis than a lower dosage (>280 vs 49 days) in a dose-finding study of patients with AIDS and CMV retinitis who had previously received anti-CMV treatment with ganciclovir, foscarnet or both drugs. In 1 of the 2 studies comparing immediate cidofovir treatment versus deferred treatment, the monthly rate of increase in retinal area affected was significantly greater with deferred treatment (1.8 or 2.2%) than with immediate treatment with the lower (0.8%) or standard (0.3%) dosage of cidofovir. None of the 3 studies demonstrated a significant difference in visual acuity or survival between the various treatment groups, although the studies were not designed with these as primary end-points.
For the 3 trials described, the standard cidofovir dosage regimen was as follows: induction dosage 5 mg/kg every week for 2 weeks; maintenance dosage 5 mg/kg every 2 weeks. The lower dosage of cidofovir included the same induction regimen followed by a maintenance dosage of 3 mg/kg every 2 weeks. Cidofovir was administered intravenously with concomitant oral probenecid and intravenous hydration to reduce the risk of nephrotoxicity.
Intraocular administration of cidofovir has been evaluated in small numbers of patients. However, it is contraindicated by the manufacturer because of a poor risk-benefit profile (see Tolerability summary section below).
Tolerability
Adverse events associated with intravenous cidofovir lead to discontinuation of therapy in approximately 20 to 25% of patients, most frequently because of nephrotoxicity. Cidofovir-induced renal impairment is dose-related and is at least partially reversible after discontinuation or interruption of therapy in most patients. Pooled tolerability data are now available from >800 patients with HIV infection, most of whom also had a diagnosis of CMV retinitis. These patients received the recommended regimen of cidofovir along with saline hydration and concomitant high dose oral probenecid to reduce the risk of dose-limiting nephrotoxicity. The most frequently reported adverse events were nephrotoxicity (22.4% of patients), uveitis/iritis (10.1%), haematological abnormalities (9.7%), nausea and/or vomiting (9.1%), fever (6.2%) and skin rash or pruritus (4.2%). It is noteworthy that postmarketing data suggest a somewhat higher incidence of anterior uveitis/iritis (occasionally with hypotony) associated with intravenous cidofovir than that reported in the pooled analysis above. In addition, concomitant administration of probenecid is frequently associated with various adverse events (e.g. nausea, emesis, fever and rash), leading to discontinuation of study treatment in about 4% of patients. Cidofovir is thought to be concentrated in renal proximal tubule cells via active transport, and probenecid is thought to reduce the concentration of cidofovir within the renal compartment.
The efficacy and tolerability profile of intraocular cidofovir was generally favourable in single centre case series of small numbers of patients with AIDS and CMV retinitis. In contrast, results of a multicentre trial showed poor efficacy. Moreover, uveitis/iritis was reported in the majority of patients and a significant proportion of patients developed clinically significant hypotony. As a result, the manufacturer abandoned further study of intraocular cidofovir and this route of administration is listed as a contraindication in the manufacturer’s product labelling for the drug.
Dosage and Administration
The recommended regimen of intravenous cidofovir in patients with CMV retinitis and AIDS who have adequate renal function is an induction dosage of 5 mg/kg weekly for 2 weeks followed by a maintenance dosage of 5 mg/kg every 2 weeks. Cidofovir is administered as a 1-hour infusion with saline hydration and concomitant oral probenecid to reduce the risk of dose-limiting nephrotoxicity. The drug is not recommended for use in patients with significantly compromised renal function. Other potentially nephrotoxic drugs (e.g. amphotericin, amino-glycosides, foscarnet, intravenous pentamidine, NSAIDs, vancomycin) should not be administered within 7 days of cidofovir treatment. Dosage adjustments or discontinuation of the drug are necessary in patients who have deterioration in renal function during cidofovir treatment. Monitoring of serum creatinine levels and urine protein is mandatory prior to each cidofovir dose; monitoring of white blood cell counts with differential, as well as intraocular pressure is also recommended during therapy with intravenous cidofovir.
Similar content being viewed by others
References
de Jong MD, Galasso GJ, Gazzard B, et al. Summary of the II International Symposium on Cytomegalovirus. Antiviral Res 1998; 39: 141–62
Baldassano V, Dunn JP, Feinberg J, et al. Cytomegalovirus retinitis and low CD4+ T-lymphocyte counts. N Engl J Med 1995; 333: 670
Kuppermann BD, Petty JG, Richman DD, et al. Correlation between CD4+ counts and prevalence of cytomegalovirus retinitis and human immunodeficiency virus-related noninfectious retinal vasculopathy in patients with acquired immunodeficiency syndrome. Am J Ophthalmol 1993; 115: 575–82
Gallant JE, Moore RD, Richman DD, et al. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated with zidovudine. J Infect Dis 1992; 166: 1223–7
Hoover DR, Saah AJ, Bacellar H, et al. Clinical manifestations of AIDS in the era of pneumocystis prophylaxis. N Engl J Med 1993; 329: 1922–6
Whitley RJ, Jacobson MA, Friedberg DN, et al. Guidelines for the treatment of cytomegalovirus diseases in patients with AIDS in the era of potent antiretroviral therapy: recommendations of an international panel. Arch Intern Med 1998 May 11; 158: 957–69
Mitchell SM, Membrey WL, Youle MS, et al. Cytomegalovirus retinitis after the initiation of highly active antiretroviral therapy: a 2 year prospective study. Br J Ophthalmol 1999; 83(6): 652–5
Martin DF, Kuppennann BD, Wolitz RA, et al. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. N Engl J Med 1999; 340(14): 1063–70
Jacobson MA. Treatment of cytomegalovirus retinitis in patients with the acquired immunodeficiencyy syndrome. N Engl J Med 1997 Jul 10; 337: 105–14
Beiser C. Recent advances: HIV infection-II. BMJ 1997 Feb 22; 314: 579–83
Masur H. Management of opportunistic infections associated with HIV infection. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. Fourth ed. v. I. New York: Churchill Livingstone, 1995: 1280–94
Masur H, Whitcup SM, Cartwright C, et al. Advances in the management of AIDS-related cytomegalovirus retinitis. Ann Intern Med 1996 Jul 15; 125: 126–36
Holland GN, Shuler JD. Progression rates of cytomegalovirus retinopathy in ganciclovir-treated and untreated patients. Arch Ophthalmol 1992; 110: 1435–42
Jabs DA. Treatment of cytomegalovirus retinitis in patients with AIDS. Ann Intern Med 1996 Jul 15; 125: 144–5
Kuppermann BD. Therapeutic options for resistant cytomegalovirus retinitis. J Acquir Immune Defic Syndrom Hum Retrovirol 1997; 14 Suppl. 1: S13–21
Caspi A. Cidofovir: a new treatment for CMV retinitis in AIDS patients. P & T 1996; 21: 462, 465–6
Ives DV Cytomegalovirus disease in AIDS. AIDS 1997 Dec; 11: 1791–7
Spector SA. Current therapeutic challenges in the treatment of cytomegalovirus retinitis. J Acquir Immune Defic Syndrom Hum Retrovirol 1997; 14 Suppl. 1: S32–5
Lea AP, Bryson HM. Cidofovir. Drugs 1996 Aug; 52: 225–30
Lalezari JP. Cidofovir: a new therapy for cytomegalovirus retinitis. J Acquir Immune Defic Syndrom Hum Retrovirol 1997; 14 Suppl. 1: S22–6
Ho HT, Woods KL, Bronson JJ, et al. Intracellular metabolism of the antiherpes agent (S)-1-[3-hydroxy-2-(phosphonyl-methoxy)propyl]cytosine. Mol Pharmacol 1992; 41: 197–202
Neyts J, Declercq E. Mechanism of action of acyclic nucleoside phosphonates against herpes virus replication. Biochem Pharmacol 1994 Jan 13; 47: 39–41
Xiong XF, Smith JL, Kim C, et al. Kinetic analysis of the interaction of cidofovir diphosphate with human cytomegalovirus DNA polymerase. Biochem Pharmacol 1996 Jun 14; 51: 1563–7
Xiong X, Smith JL, Chen MS. Effect of incorporation of cidofovir into DNA by human cytomegalovirus DNA polymerase on DNA elongation. Antimicrob Agents Chemother 1997 Mar; 41: 594–9
Physicians’ Desk Reference. 53rd ed. Montvale (NJ): Medical Economics Company, Inc, 1999
Cherrington JM, Allen SJ, McKee BH, et al. Kinetic analysis of the interaction between the diphosphate of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, ddCTP, AZTTP, and FIAUTP with human DNA polymerases β and γ. Biochem Pharmacol 1994; 48(10): 1986–8
Andrei G, Snoeck R, Schols D, et al. Comparative activity of selected antiviral compounds against clinical isolates of human cytomegalovirus. European Journal of Clinical Micorbiology and Infectious Diseases 1991; 10(12): 1026–33
De Clercq E, Sakuma T, Baba M, et al. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res 1987; 8: 261–72
Neyts J, Snoeck R, Balzarini J, et al. Particular characteristics of the anti-human cytomegalovirus activity of (S)-l-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) in vitro. Antiviral Res 1991 Jul; 15: 41–52
Otova B, Votruba I, Holy A. Pretreatment of the host cell with l-(S)-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) is sufficient for its antiviral effect. Acta Virol 1992 May; 36: 313–9
Shigeta S, Konno K, Baba M, et al. Comparative inhibitory effects of nucleoside analogues on different clinical isolates of human cytomegalovirus in vitro. J Infect Dis 1991; 163: 270–5
Kendle JB, Fan-Havard P. Cidofovir in the treatment of cytomegaloviral disease. Ann Pharmacother 1998 Nov; 32: 1181–92
Neyts J, Snoeck R, Schols D, et al. Selective inhibition of human cytomegalovirus DNA synthesis by (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine [(S)-HPMPC] and 9-(l,3-dihydroxy-2-propoxymethyl)guanine (DHPG). Virology 1990; 179: 41–50
Manion DJ, Vibhagool A, Chou T-C, et al. Susceptibility of human cytomegalovirus to two-drug combinations in vitro. Antiviral Ther 1996 Dec; 1: 237–45
Mulato AS, Cherrington JM, Chen MS. Anti-HCMV activity of cidofovir in combination with antiviral compounds and immunosuppressive agents: in-vitro analyses. Antiviral Chem Chemother 1996 Jul; 7: 203–8
Snoeck R, Andrei G, Schols D. Activity of different antiviral drug combinations against human cytomegalovirus replication in vitro. Eur J Clin Microbiol Infect Dis 1992 Dec; 11: 1144–55
Cherrington JM, Mulato AS, Fuller M, et al. In vitro selection of a human cytomegalovirus that is resistant to cidofovir [abstract]. 35th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1995 Sep 17; San Francisco, 200
Snoeck R, Andrei G, De Clercq E. Patterns of resistance and sensitivity to antiviral compounds of drug-resistant strains of human cytomegalovirus selected in vitro. Eur J Clin Microbiol Infect Dis 1996 Jul; 15: 574–9
Cherrington JM, Allen SJW, Mulato AS, et al. Sensitivities of human cytomegalovirus clinical isolates to cidofovir [abstract]. Antiviral Res 1995 Mar; 26: A319
Sullivan V, Biron KK, Talarico C, et al. A point mutation in the human cytomegalovirus DNA polymerase gene confers resistance to ganciclovir and phosphonylmethoxyalkyl derivatives. Antimicrob Agents Chemother 1993 Jan; 37: 19–25
Cherrington JM, Smith IL, Jiles RE, et al. Ganciclovir resistant CMV: implications of UL97 and polymerase mutations in cross resistance to cidofovir [abstract no. 88]. Antiviral Res 1996; 30(1): A44
Erice A, Gil-Roda C, Pérez J-L, et al. Antiviral susceptibilities and analysis of UL97 and DNApolymerase sequences of clinical cytomegalovirus isolates from immunocompromised patients. J Infect Dis 1997 May; 175: 1087–92
Stanat SC, Reardon JE, Erice A, et al. Ganciclovir-resistant cytomegalovirus clinical isolates: mode of resistance to ganciclovir. Antimicrob Agents Chemother 1991 Nov; 35: 2191–7
Tatarowicz WA, Lurain NS, Thompson KD. A ganciclovir-resistant clinical isolate of human cytomegalovirus exhibiting cross-resistance to other DNA polymerase inhibitors. J Infect Dis 1992 Oct; 166: 904–7
Smith IL, Cherrington JM, Jiles RE, et al. High-level resistance of cytomegalovirus to ganciclovir is associated with alterations in both the UL97 and DNA polymerase genes. J Infect Dis 1997 Jul; 176: 69–77
Cherrington JM, Fuller MD, Lamy PD, et al. In vitro antiviral susceptibilities of isolates from cytomegalovirus retinitis patients receiving first- or second-line cidofovir therapy: relationship to clinical outcome. J Infect Dis 1998 Dec; 178: 1821–5
Snoeck R, Andrei G, De Clercq E. Human cytomegalovirus (HCMV) strains selected under selective pressure of phosphonoformate (PFA) are resistant for both PFA and phosphonylmethoxyethyl (PME) derivatives in vitro [abstract no. 177]. Antiviral Res 1995; 26(3): A320
Baldanti F, Underwood MR, Stanat SC, et al. Single amino acid changes in the DNA polymerase confer foscarnet resistance and slow-growth phenotype, while mutations in the UL97-encoded phosphotransferase confer ganciclovir resistance in three double-resistant human cytomegalovirus strains recovered from patients with AIDS. J Virol 1996 Mar; 70: 1390–5
Sullivan V, Coen DM. Isolation of foscarnet-resistant human cytomegalovirus patterns of resistance and sensitivity to other antiviral drugs. J Infect Dis 1991 Oct; 164: 781–4
Chou S, Marousek G, Guentzel S, et al. Evolution of mutations conferring multidrug resistance during prophylaxis and therapy for cytomegalovirus disease. J Infect Dis 1997 Sep; 176: 786–9
Besen G, Flores-Aguilar M, Assil KK, et al. Long-term therapy for herpes retinitis in an animal model with high-concentrated liposome-encapsulated HPMPC. Arch Ophthalmol 1995 May; 113: 661–8
Flores-Aguilar M, Huang J-S, Wiley CA, et al. Long-acting therapy of viral retinitis with (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine. J Infect Dis 1994 Mar; 169: 642–7
Kuppermann BD, Assil KK, Vuong C, et al. Liposome-encapsulated (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine for long-acting therapy of viral retinitis. J Infect Dis 1996 Jan; 173: 18–23
Kern ER, Palmer J, Hartline C, et al. Comparison of efficacy and toxicity of HPMPC and cyclic HPMPC in animal models for severe herpesvirus infections [abstract no. 195]. Antiviral Res 1995 Mar; 26(3): A329
Cundy KC. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin Pharmacokinet 1999 Feb;36: 127–43
Cundy KC, Petty BG, Flaherty J, et al. Clinical pharmacokinetics of cidofovir in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother 1995 Jun; 39: 1247–52
Wachsman M, Petty BG, Cundy KC, et al. Pharmacokinetics, safety and bioavailability of HPMPC (cidofovir) in human immunodeficiency virus-infected subjects. Antiviral Res 1996; 29: 153–61
Lalezari JP, Drew WL, Glutzer E, et al. (S)-1-[3-Hydroxy-2-(phosphonylmethoxy)propyl]cytosine (cidofovir): results of a phase I/II study of a novel antiviral nucleotide analogue. J Infect Dis 1995 Apr; 171: 788–96
Lalezari J, Holland G, Stagg R, et al. A randomized controlled study of cidofovir (CDV) for relapsing cytomegalovirus retinitis (CMV-R) in patients with AIDS [abstract]. 35th International Conference on Antimicrobial Agents and Chemotherapy; 1995 Sep 17–20 Program Addendum; San Francisco, 9
Lalezari JP, Stagg RJ, Kuppennann BD, et al. Intravenous cidofovir for peripheral cytomegalovirus retinitis in patients with AIDS. Ann Intern Med 1997 Feb 15; 126: 257–63
Ocular Complications of AIDS Research Group. Parenteral cidofovir for cytomegalovirus retinitis in patients with AIDS: the HPMPC Peripheral Cytomegalovirus Retinitis Trial: a randomized, controlled trial. Ann Intern Med 1997 Feb 15; 126: 264–74
Lalezari JP, Holland GN, Kramer F, et al. Randomized, controlled study of the safety and efficacy of intravenous cidofovir for the treatment of relapsing cytomegalovirus retinitis in patients with AIDS. J Acquir Immune Defic Syndrom Hum Retrovirol 1998 Apr 1; 17: 339–44
Polis MA, Spooner KM, Baird BF, et al. Anticytomegaloviral activity and safety of cidofovir in patients with human immunodeficiency virus infection and cytomegalovirus viruria. Antimicrob Agents Chemother 1995 Apr; 39: 882–6
Podlasin R, Giziñska J, Szymczak J, et al. The impact of highly active antiretroviral therapy on the course of CMV retinitis after the interruption of anti-CMV therapy [abstract]. AIDS 1998 Nov; 12 Suppl. 4: S89
Lieberman R, Orellana J, Roseman JB. Prolonged dosing schedules using cidofovir in patients with CMV retinitis [abstract]. 12th World AIDS Conference; 1998 June 28–July 3; Geneva, 568
Jacobson MA, Wilson S, Stanley H, et al. Phase I study of combination therapy with intravenous cidofovir and oral ganciclovir for cytomegalovirus retinitis in patients with AIDS. Clin Infect Dis 1999; 28: 528–33
Kirsch LS, Arevalo JF, De Clercq E, et al. Phase MI study of intravitreal cidofovir for the treatment of cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. Am J Ophthalmol 1995 Apr; 119: 466–76
Kirsch LS, Arevalo JF, Chavez de la Paz E, et al. Intravitreal cidofovir (HPMPC) treatment of cytomegalovirus retinitis in patients with acquired immune deficiency syndrome. Ophthalmology 1995 Apr; 102: 533–43
Rahhal FM, Arevalo JF, Munguia D, et al. Intravitreal cidofovir for the maintenance treatment of cytomegalovirus retinitis. Ophthalmology 1996 Jul; 103: 1078–83
Taskintuna I, Rahhal FM, Arevalo JF, et al. Low-dose intravitreal cidofovir (HPMPC) therapy of cytomegalovirus retinitis in patients with acquired immune deficiency syndrome. Ophthalmology 1997 Jun; 104: 1049–57
Kuppermann B, Wolitz R, Stagg R, et al. A phase II randomized, double-masked study of (IO) intraocular cidofovir for relapsing cytomegalovirus retinitis (CMV-R) in patients with AIDS [abstract]. Vitreous Society Annual Meeting, Cancun, Mexico, December 8–12, 1996
A phase II randomized, double-masked study of intraocular cidofovir for relapsing cytomegalovirus retinitis in patients with AIDS. Data on file, Gilead Sciences, Inc., Foster City, CA, USA
Piadena R, Francom S, Postma D, et al. Safety results from the cidofovir (Vistide) Expanded Access Program [abstract]. AIDS 1998 Nov; 12 Suppl. 4: S90
Gilead Vistide. ‘Dear doctor’ letter underscores need for renal monitoring. FDC Pink 1998 Aug 24; 60: 10
Skiest DJ, Duong M, Park S, et al. Complications of therapy with intravenous cidofovir: severe nephrotoxicity and anterior uveitis. Infect Dis Clin Pract 1999; 8: 151–7
Schliefer K, Rockstroh JK, Spengler U. Nephrogenic diabetes insipidus in a patient taking cidofovir. Lancet 1997 Aug 9; 350: 413–4
Davis JL, Taskintuna I, Freeman WR, et al. Iritis and hypotony after treatment with intravenous cidofovir for cytomegalovirus retinitis (see comments). Arch Ophthalmol 1997 Jun; 115: 733–7
Akler ME, Johnson DW, Burman WJ, et al. Anterior uveitis and hypotony after intravenous cidofovir for the treatment of cytomegalovirus retinitis. Ophthalmology 1998 Apr; 105: 651–7
Friedberg DN. Hypotony and visual loss with intravenous cidofovir treatment of cytomegalovirus retinitis (letter) (see comments). Arch Ophthalmol 1997 Jun; 115: 801–2
MacRae K, McCluskey P. Safety profile of cidofovir in a patient group with heavily pre-treated CMV disease [abstract]. AIDS 1998 Nov; 12 Suppl. 4: S86
Malecha MA, Walton RC. Characteristics of anterior uveitis associated with intravenous cidofovir in patients with cytomegalovirus retinitis [abstract]. South Med J 1998 Oct; 91: S69
Neau D, Renaud-Rougier MB, Viallard JF, et al. Intravenous cidofovir-induced iritis. Clin Infect Dis 1999 Jan; 28: 156–7
Palau LA, Tufty GT, Pankey GA. Recurrent iritis after intravenous administration of cidofovir. Clin Infect Dis 1997 Aug; 25: 337–8
Taskintuna I, Rahhal FM, Rao NA, et al. Adverse events and autopsy findings after intravitreous cidofovir (HPMPC) therapy in patients with acquired immune deficiency syndrome (AIDS). Ophthalmology 1997 Nov; 104: 1827–36
Protocol GS-503. Data on file, Gilead Sciences, Inc., Foster City, CA, USA
Hardy WD. Management strategies for patients with cytomegalovirus retinitis. J Acquir Immune Defic Syndrom Hum Retrovirol 1997; 14 Suppl. 1: S7–S12
Jacobson MA. AIDS-related cytomegalovirus retinitis. Drugs Today 1998 May; 34: 409–13
Polis MA. Cidofovir [commentary]. Drugs 1996 Aug; 52: 231
Meenken C, for the Fomiversen Clinical Trials Group. Advantages of a new local therapy, fomiversen, for the treatment of CMV retinits [abstract no. P269]. AIDS 1998; 12 Suppl. 4: S84
Perry CM, Barman Balfour JA. Fomivirsen. Drugs 1999; 57: 375–80
Malinverni R, Oggier W, Steger PJK, et al. Pharmacoeconomic analysis of four treatment options for CMV retinitis in Swiss AIDS patients [abstract]. 12th World AIDS Conference; 1998 Jun 28–Jul 3; Geneva, 1068
Lee TA, Sullivan SD, Ramsey SD, et al. Economic evaluation of systemic treatments for CMV retinitis in persons with AIDS in Switzerland [abstract no. PID3]. Value in Health 1999; 2(3): 183
Rockstroh J, Jäger H, Schliefer K, et al. Clinical practice of CMV retinitis treatment with cidofovir in experienced patients: results of the German Cidofovir Study Group [abstract]. 12th AIDS 1998 Jun 28: 313
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: B.G. Gazzard, Chelsea & Westminster Healthcare National Health Service Trust, Chelsea & Westminster Hospital, London, England; M.A. Jacobson, UCSF AIDS Program, San Francisco General Hospital, San Francisco, California, USA; Y. Khaliq, Clinical Investigation Unit, The Ottawa Hospital, Ottawa, Ontario, Canada; J.P. Lalezari, University of California, Mt Zion Medical Center, San Francisco, California, USA; M.A. Polis, Intramural AIDS Program, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA; J. Rockstroh, Department of Medicine, University of Bonn, Bonn, Germany; G.J. van den Horn, Department of Ophthalmology, University of Amsterdam, Amsterdam, The Netherlands; R.J. Whitley, The University of Alabama at Birmingham, Department of Pediatrics, Division of Clinical Virology, Children’s Hospital, Birmingham, Alabama, USA.
Data Selection
Sources: Medical literature published in any language since 1966 on cidofovir, identified using AdisBase (a proprietary database of Adis International, Auckland, New Zealand), Medline and EMBASE. Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: AdisBase search terms were ‘Cidofovir’ or ‘GS-0502’ or ‘GS-504’ or ‘HPMPC’. Medline search terms were ‘Cidofovir’ or ‘GS-0502’ or ‘GS-504’ or ‘HPMPC’. EMBASE search terms were ‘Cidofovir’ or ‘GS-0502’ or ‘GS-504’ ‘HPMPC’. Searches were last updated 5 July 1999.
Selection: Studies in patients with cytomegalovirus retinitis who received cidofovir. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Cidofovir, cytomegalovirus retinitis, antiviral, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability, dosage and administration.
Rights and permissions
About this article
Cite this article
Plosker, G.L., Noble, S. Cidofovir. Drugs 58, 325–345 (1999). https://doi.org/10.2165/00003495-199958020-00015
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199958020-00015