Summary
Abstract
Protease inhibitor boosting involves concurrent administration of a protease inhibitor, such as saquinavir, plus a potent inhibitor of cytochrome P450 (CYP) 3A4, usually ritonavir in subtherapeutic doses. Since protease inhibitors are extensively metabolised by CYP3A4, this results in a marked increase in systemic exposure of saquinavir or other protease inhibitors boosted by ritonavir. As with traditional protease inhibitor regimens, boosted regimens are typically used in combination with nucleoside reverse transcriptase inhibitors (NRTIs).
In protease inhibitor-experienced and -naive patients with HIV infection, twice-daily and once-daily boosted saquinavir regimens achieved good rates of viral suppression, improved CD4+ cell counts and were generally well tolerated in clinical trials. Encouraging results have also been reported in a number of small studies in heavily pretreated HIV-infected patients who received salvage therapy comprising double-boosted regimens of saquinavir plus lopinavir with subtherapeutic doses of ritonavir, along with other agents.
The largest clinical trials have been multicentre, randomised comparisons of twice-daily boosted saquinavir versus twice-daily boosted indinavir (MaxCminl) or lopinavir (MaxCmin2) regimens. In the MaxCmin1 study, >90% of patients in both groups had an undetectable viral load (<400 copies/mL) after 48 weeks of therapy in the on-treatment analysis. However, viral suppression was achieved in significantly more saquinavir than indinavir recipients in the intention-to-treat analysis, which appeared to be due to the significantly greater percentage of patients in the indinavir group who switched from randomised therapy because of adverse events. Interim 24-week results of the MaxCmin2 trial indicate that 90% of patients in both groups combined had plasma HIV RNA levels <400 copies/ mL; final results at 48 weeks will report data separately for the boosted regimens of saquinavir and lopinavir.
Conclusion: Boosted protease inhibitor regimens (including two NRTIs) are recommended as a first-line option in current HIV treatment guidelines and are used extensively in clinical practice. The convenient administration schedule and good pharmacokinetic profile associated with boosted saquinavir regimens have the potential to increase adherence to therapy and improve antiretroviral effects through increased drug exposure. Twice-daily boosted saquinavir is one of the most extensively evaluated boosted protease inhibitor regimens and has been shown to have good efficacy on surrogate markers of HIV disease as well as significant tolerability advantages over boosted indinavir. Once-daily boosted saquinavir regimens may be most suitable for HIV-infected patients with busy lifestyles and those who would benefit from directly observed therapy.
Overview of Pharmacodynamic Properties
Inhibition of human HIV protease by saquinavir results in the formation of immature, noninfectious virus particles. In vitro studies have shown that saquinavir is highly active against HIV in human cells that are acutely or chronically infected with the virus. When saquinavir was combined with one or two other antiretrovirals, including nucleoside reverse transcriptase inhibitors (NRTIs) and/or the non-nucleoside reverse transcriptase inhibitor (NNRTI) nevirapine, additive to synergistic activity against HIV was demonstrated in vitro without cytotoxic effects in host cells. Synergistic activity was also shown when saquinavir was combined with lopinavir, whereas additive effects were generally shown when other protease inhibitors were combined with lopinavir. The principal protease gene mutations that confer resistance to saquinavir are L90M and G48V. Resistance to saquinavir is dependent upon both the specific mutations present and the number of mutations present. Studies have shown that patients whose virus contains up to four or five mutations in protease are still likely to achieve a clinical response with boosted saquinavir (saquinavir soft-gel capsule [SGC] 1000mg plus ritonavir 100mg twice daily and NRTIs). Cross resistance occurs between saquinavir and other protease inhibitors, but is variable and often difficult to predict.
Pharmacokinetics of Saquinavir in Boosted Regimens
Protease inhibitor boosting (or pharmacoenhancement) typically involves the use of subtherapeutic doses of ritonavir, a potent inhibitor of cytochrome P450 (CYP) 3A4, administered concurrently with therapeutic doses of other protease inhibitors such as saquinavir, which are metabolised by CYP3A4. When boosted saquinavir regimens are administered to healthy volunteers or patients with HIV infection, systemic exposure to saquinavir is increased several-fold, generally without an increase in adverse events. In comparative studies of boosted versus nonboosted regimens of saquinavir SGC, mean area under the concentration-time curve (AUC) of saquinavir was typically about 5-fold (range 3-to 9-fold) greater with boosted regimens; trough (Cmin) and peak plasma concentrations of saquinavir were usually increased by a similar magnitude. Inter-individual variability in saquinavir kinetics, although still substantial, is reduced when the drug is used in boosted regimens. In dose-ranging pharmacokinetic interaction studies in which healthy volunteers received saquinavir SGC and ritonavir concurrently, ritonavir doses of 100mg once daily to 400mg twice daily had broadly similar effects at increasing systemic exposure to saquinavir.
In general, pharmacokinetic analyses conducted with boosted saquinavir regimens have shown wide interpatient variation in the pharmacokinetics of saquinavir, although saquinavir Cmin values were consistently above 50 μg/L and/ or the 90% or 95% inhibitory concentration (IC90 or IC95) against HIV. Saquinavir, like most protease inhibitors, is highly bound to plasma proteins, and adjustment for plasma protein binding is required when determining ratios such as Cmin/IC. The majority of pharmacokinetic analyses in adults with HIV infection have evaluated saquinavir SGC 1000mg plus ritonavir 100mg twice daily; saquinavir SGC 1600mg plus ritonavir 100mg once daily; and double-boosted regimens (e.g. saquinavir SGC 1000mg plus lopinavir/ritonavir twice daily). Patients also received at least two NRTIs/NNRTIs.
In nonboosted regimens, the relative bioavailability of saquinavir hard-gel capsule (HGC) is several-fold lower than that of saquinavir SGC. However, in healthy volunteers and patients with HIV infection, boosted regimens with saquinavir HGC achieved similar or greater systemic exposure of saquinavir compared with boosted regimens using saquinavir SGC.
In double-boosted regimens (therapeutic doses of saquinavir and lopinavir plus subtherapeutic doses of ritonavir, usually with two or more NRTIs) in patients with HIV infection, the addition of saquinavir to lopinavir/ritonavir therapy had minimal effect on the plasma concentrations of these protease inhibitors. Other pharmacokinetic drug interaction studies in healthy volunteers or patients with HIV infection found no clinically significant interactions between boosted saquinavir regimens at steady state and single-dose didanosine or multiple doses of efavirenz or the HIV-1 fusion inhibitor enfuvirtide. The addition of rifampicin (rifampin) decreased the AUC of saquinavir by approximately 50% at steady state in HIV-infected patients with tuberculosis receiving a once-daily boosted regimen of saquinavir. In general, concurrent ritonavir administration also appears to preserve saquinavir exposure in the presence of the enzyme inducer rifabutin, although saquinavir/ritonavir dosages used in boosted regimens have not been evaluated.
Therapeutic Use of Saquinavir in Boosted Regimens
Clinical efficacy results from studies evaluating boosted or double-boosted regimens of saquinavir in adult patients with HIV infection have focused on changes in plasma HIV RNA levels. In addition, modest improvements in CD4+ cell counts were observed with boosted or double-boosted saquinavir regimens across all of the studies.
Twice-daily regimens: All clinical trials of twice-daily boosted saquinavir regimens have used saquinavir (SGC or HGC) 1000mg plus ritonavir 100mg twice daily with two or more NRTIs/NNRTIs. Comparator boosted protease inhibitor regimens also included two or more NRTIs/NNRTIs.
In the MaxCmin1 study, a multicentre randomised comparison between twice-daily boosted saquinavir SGC and boosted indinavir (indinavir/ritonavir 800/100mg twice daily) in 306 protease inhibitor-experienced or -naive patients, significantly more saquinavir than indinavir recipients had an undetectable viral load (plasma HIV RNA <400 copies/mL) at 48 weeks in the intention-to-treat (ITT) analysis (68% vs 53%, p = 0.014). The difference appeared to be the result of significantly fewer patients in the boosted saquinavir group who switched therapy because of adverse effects (a switch was considered to be a treatment failure in the ITT analysis). The on-treatment (OT) analysis at 48 weeks showed that both regimens were similarly effective at achieving an undetectable viral load (94% vs 90%).
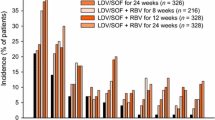
Final results are not yet available from the MaxCmin2 trial, a large (n = 326) randomised multicentre study comparing twice-daily boosted saquinavir SGC versus boosted lopinavir (lopinavir/ritonavir 400/100mg twice-daily) regimens in a mixed patient population. Interim 24-week results combining data from patients in both treatment arms showed that 77% and 90% of patients had an undetectable viral load (<400 copies/mL) in the ITT and OT analyses, respectively.
In both MaxCmin1 and MaxCmin2, saquinavir was switched from the SGC to the HGC formulation if adverse gastrointestinal disturbances occurred.
In general, the clinical efficacy demonstrated with twice-daily boosted saquinavir in comparative trials is supported by data from noncomparative studies in protease inhibitor-experienced patients.
Once-daily regimens: All clinical trials with once-daily boosted saquinavir regimens have used the SGC formulation of saquinavir, and most have used a regimen of saquinavir SGC 1600mg plus ritonavir 100mg once daily with two NRTIs. This was the regimen used in the largest trial (FOCUS), which compared boosted saquinavir versus efavirenz 600mg once daily plus two NRTIs in 152 antiretroviral-naive patients. In general, the saquinavir and efavirenz regimens were similarly effective at achieving an undetectable viral load (<400 copies/mL) at 48 weeks in both the ITT (59% vs 73%) and the OT analysis (83% vs 93%), although statistical analysis was not provided in the abstract report. Interim data at 24 weeks showed that, at the <50 copies/mL level, there was a significant difference favouring the efavirenz group (60% vs 78%, p = 0.008).
In the largest noncomparative trial with once-daily boosted saquinavir (HIVNAT 001.3), 69 patients who had an undetectable viral load (<50 copies/mL) for 2 years while receiving saquinavir SGC 1400mg twice daily plus two NRTIs had their saquinavir SGC regimen switched to 1600mg once daily given concurrently with ritonavir 100mg once daily. After 48 weeks in the ITT analysis, 91% of patients maintained an undetectable viral load, and this was accompanied by a statistically significant improvement in CD4+ cell count. Other, smaller noncomparative trials with once-daily boosted saquinavir regimens have primarily been conducted in patients with prior exposure to protease inhibitors. In most of these trials an undetectable viral load was achieved in the majority of patients at interim or final analysis.
Double-boosted regimens: Although several studies have been conducted with double-boosted saquinavir regimens as salvage therapy, none has evaluated more than 50 patients. The most frequently used regimen has been saquinavir SGC 1000mg twice daily plus lopinavir/ritonavir 400mg/100mg twice daily, usually in combination with two or more NRTIs, and patients typically had HIV infection resistant to all three drug classes. In general, results have been encouraging. In a representative study in 46 patients with multiclass drug resistance, approximately one-third to one-half of patients had an undetectable viral load (<80 copies/mL) in the ITT analysis at 24 weeks. In approximately half of the patients, the double-boosted regimen was preceded by a 3-month antiretroviral drug holiday; results in this group were similar to those in patients whose antiretroviral therapy was not temporarily interrupted. In a nonrandomised single-centre study (n = 42), double-boosted saquinavir achieved plasma HIV RNA levels <500 copies/mL in 39% of patients at 48 weeks in the ITT analysis compared with 21% of those who received a double-boosted amprenavir regimen (statistical analysis not provided). In these and other studies with double-boosted saquinavir regimens, somewhat better results were demonstrated in OT than ITT analyses, as would be expected.
Tolerability of Boosted Saquinavir
In general, saquinavir in boosted or double-boosted regimens was well tolerated in clinical trials of up to 48 weeks’ duration in adult patients with HIV infection. The vast majority of trials involved concomitant use of at least two NRTIs (some regimens included NNRTIs).
Twice-daily regimens: In the MaxCmin1 trial, there was a significantly lower rate of withdrawal from the study (28% vs 42%, p = 0.025) and withdrawal because of adverse events (15% vs 28%, p = 0.006) with saquinavir than indinavir in twice-daily boosted regimens over 48 weeks. In the MaxCmin2 trial, 24-week data showed that the tolerability profile of twice-daily boosted saquinavir was similar to that of a twice-daily boosted lopinavir regimen. Saquinavir was initially administered as the SGC formulation in both MaxCmin1 and MaxCmin2 but was switched to the HGC formulation for patients who experienced gastrointestinal disturbances.
Once-daily regimens: During the 48-week HIVNAT 001.3 trial, none of the 69 patients (who had switched from a traditional saquinavir SGC-containing antiretroviral regimen to a once-daily boosted saquinavir SGC regimen) discontinued therapy because of adverse events. Severe (grade 3 or 4) adverse events (4%) and laboratory abnormalities (12%) were reported during the first 24 weeks of the trial, and lipodystrophy (a common adverse effect of antiretroviral therapy) was newly reported in 19% of patients. During the 24-week period before and after switching to the boosted regimen, a significant reduction in nausea and vomiting was reported following the switch to the boosted regimen.
In the FOCUS trial comparing once-daily boosted saquinavir SGC versus efavirenz (both with two NRTIs), moderate to severe nausea (22.2% vs 3.8%) and vomiting (6.2% vs 0%) were more frequently reported in the saquinavir group at 48 weeks, and more patients in the saquinavir group discontinued therapy because of adverse gastrointestinal events. Although a modest increase in serum lipid levels is commonly reported with boosted saquinavir (and other antiretroviral) regimens, the FOCUS trial found that the strongest predictor of increases in serum triglyceride and cholesterol levels was concurrent treatment with stavudine as one of the NRTIs.
Double-boosted regimens: Half of the 28 patients who received double-boosted therapy in the PIE study required temporary dosage reductions of saquinavir SGC, primarily because of gastrointestinal disturbances. Saquinavir SGC dosage was gradually increased back to the target level of 1000mg twice daily in all 14 patients. Grade 3 or 4 diarrhoea occurred in 18% of patients, myalgia/arthralgia in 8% and nausea in 7%. In this and a similar-sized study of double-boosted saquinavir, approximately 10% of patients withdrew from therapy because of adverse events. Interestingly, no significant gastrointestinal adverse effects were noted in the similar-sized trial.
Dosage and Administration
In Europe, saquinavir (SGC or HGC) 1000mg plus ritonavir 100mg twice daily is approved for use in HIV infection, and an application for this boosted regimen is pending in the US. Once-daily boosted saquinavir regimens are not yet approved in Europe or the US for saquinavir SGC or HGC. In clinical trials with boosted or double-boosted saquinavir regimens, the most frequently used combinations have been as follows: saquinavir SGC or HGC 1000mg twice daily plus ritonavir 100mg twice daily (with at least two NRTIs/NNRTIs); saquinavir SGC 1600mg once daily plus ritonavir 100mg once daily (in combination with at least two NRTIs); and saquinavir 1000mg SGC twice daily plus lopinavir and ritonavir 400/ 100mg twice daily (in combination with at least two NRTIs).






Similar content being viewed by others
Notes
Tradenames are included for identification purposes and do not imply product endorsement.
References
Figgitt DP, Plosker GL. Saquinavir soft-gel capsule: an updated review of its use in the management of HIV infection. Drags 2000 Aug; 60(2): 481–516
Perry CM, Noble S. Saquinavir soft-gel capsule formulation: a review of its use in patients with HIV infection. Drags 1998 Mar; 55(3): 461–86
Noble S, Faulds D. Saquinavir: a review of its pharmacology and clinical potential in the management of HIV infection. Drugs 1996; 52(1): 93–112
Moyle GJ. Saquinavir-soft gel: establishing saquinavir in HAART regimens. Expert Opin Invest Drags 1998; 7(8): 1313–22
Gill J, Feinberg J. Saquinavir soft gelatin capsule: a comparative safety review. Drug Saf 2001; 24(3): 223–32
Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N Engl J Med 1997 Sep 11; 337: 725–33
Cameron DW, Heath-Chiozzi M, Danner S, et al. Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. Advanced HIV Disease Ritonavir Study Group. Lancet 1998 Feb 21; 351(9102): 543–9
Haubrich R, Lalezari J, Follansbee SE, et al. Improved survival and reduced clinical progression in HIV-infected patients with advanced disease treated with saquinavir plus zalcitabine. Antiviral Ther 1998; 3: 33–42
Moyle GJ, Back D. Principles and practice of HIV-protease inhibitor pharmacoenhancement. HIV Med 2001 Apr; 2(2): 105–13
British HIV Association (BHIVA). British HIV Association Guidelines [online]. Available from URL: http://www.bhiva.org/guidelines.htm [Accessed 2003 Feb 20]
Moyle G. Use of HIV protease inhibitors as pharmacoenhancers. AIDS Reader 2001; 11(2): 87–98
Malaty LI, Kuper JJ. Drug interactions of HIV protease inhibitors. Drug Saf 1999 Feb; 20: 147–69
von Moltke LL, Greenblatt DJ, Grassi JM, et al. Protease inhibitors as inhibitors of human cytochromes P450: high risk associated with ritonavir. J Clin Pharmacol 1998; 38: 106–11
Eagling VA, Back DJ, Barry MG. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br J Clin Pharmacol 1997 Aug; 44(2): 190–4
Hoffmann-La Roche Inc. Fortovase® (saquinavir) soft gelatin capsules —complete product information [online]. Available from URL: http://www.rocheusa.com/products/fortovase/pi.html [Accessed 2002 Oct 30]
Molla A, Mo H, Vasavanonda S, et al. In vitro antiviral interaction of lopinavir with other protease inhibitors. Antimicrob Agents Chemother 2002 Jul; 46(7): 2249–53
Para MF, Glidden DV, Coombs RW, et al. Baseline human immunodeficiency virus type 1 phenotype, genotype, and RNA response after switching from long-term hard-capsule saquinavir to indinavir or soft-gel-capsule saquinavir in AIDS Clinical Trials Group protocol 333. J Infect Dis 2000 Sep; 182(3): 733–43
Schmidt B, Walter H, Moschik B, et al. Simple algorithm derived from a geno-/phenotypic database to predict HIV-1 protease inhibitor resistance. AIDS 2000; 14: 1731–8
Valer L, de Mendoza C, Gonzalez de Requena D, et al. Impact of HIV genotyping and drag levels on the response to salvage therapy with saquinavir/ritonavir. AIDS 2002; 16(14): 1964–6
Barreiro P, Camino N, de Mendoza C, et al. Comparison of the efficacy, safety and predictive value of HIV genotyping using distinct ritonavir-boosted protease inhibitors. Int J Antimicrob Agents 2002; 20: 438–43
Kemper CA, Witt MD, Keiser PH, et al. Sequencing of protease inhibitor therapy: insights from an analysis of HIV phenotypic resistance in patients failing protease inhibitors. AIDS 2001; 15: 609–15
Hsu A, Granneman GR, Cao G. Pharmacokinetic interactions between two human immunodeficiency virus protease inhibitors, ritonavir and saquinavir. Clin Pharmacol Ther 1998; 63(4): 453–64
Merry C, Barry MG, Mulcahy F, et al. Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients. AIDS 1997 Mar 15; 11(4): F29–33
Buss N, Snell P, Bock J, et al. Saquinavir and ritonavir pharmacokinetics following combined ritonavir and saquinavir (soft gelatin capsules) administration. Br J Clin Pharmacol 2001; 52: 255–64
Gisolf EH, van Heeswijk RPG, Hoetelmans RWM, et al. Decreased exposure to saquinavir in HIV-1-infected patients after long-term antiretroviral therapy including ritonavir and saquinavir. AIDS 2000; 14: 801–5
Kilby JM, Sfakianos G, Gizzi N, et al. Safety and pharmacokinetics of once-daily regimens of soft-gel capsule saquinavir plus minidose ritonavir in human immunodeficiency virus-negative adults. Antimicrob Agents Chemother 2000; 44(10): 2672–8
Kurowski M, Müller M, Donath F, et al. Single daily doses of saquinavir achieve HIV-inhibitory concentrations when combined with baby-dose ritonavir. Eur J Med Res 1999 Mar 26; 4(3): 101–4
Kilby JM, Hill A, Buss N. The effect of ritonavir on saquinavir plasma concentration is independent of ritonavir dosage: combined analysis of pharmacokinetic data from 97 subjects. HIV Medicine 2002 Apr; 3(2): 97–104
Cardiello PG, van Heeswijk RP, Hassink EA, et al. Simplifying protease inhibitor therapy with once-daily dosing of saquinavir soft-gelatin capsules/ritonavir (1600/100 mg): HIVNAT 001.3 study. J Acquir Immune Defic Syndr 2002 Apr 15; 29(5): 464–70
Hoffmann-La Roche Inc. Invirase® (saquinavir mesylate) capsules —complete product information [online]. Available from URL: http://www.rocheusa.com/products/invirase/pi.html [Accessed 2002 Oct 30]
Kurowski M, Sternfeld T, Hill A, et al. Comparative pharmacokinetics and short-term safety of Fortovase/ritonavir and Invirase/ritonavir 1000mg/100mg bid [abstract/poster no. S29e]. 9th Conference on Retroviruses and Opportunistic Infections; 2002 Feb 24–28; Seattle
Cardiello P, Ananworanich J, Monhaphol T, et al. Pharmacokinetics of once daily saquinavir-hard gel caps and saquinavir-soft gel caps boosted with ritonavir in HIV-1+ Thai patients [abstract no. MoPpB2007]. XIV International AIDS Conference; 2002 Jul 7–12; Barcelona, 32
Kurowski M, Sternfeld T, Sawyer AW, et al. Minor interaction between efavirenz and saquinavir, when combined with small doses of ritonavir in a q.d. regimen. Roche, 2002. (Data on file)
Piketty C, Race E, Castiel P, et al. Efficacy of a five-drug combination including ritonavir, saquinavir and efavirenz in patients who failed on a conventional triple-drug regimen: phenotypic resistance to protease inhibitors predicts outcome of therapy. AIDS 1999 Jul 30; 13(11): F71–7
Kurowski M, Arslan A, Arasteh K, et al. Comparative pharmacokinetics of twice daily (BID) Fortovase/ritonavir and Invirase/ritonavir [poster no. FOR062]. 8th Deutscher AIDS Kongress; 2002 Jul 4–7; Berlin
vanHeeswijk RPG, Veldkamp AI, Mulder JW, et al. Once-daily dosing of saquinavir and low-dose ritonavir in HIV-1-infected individuals: A pharmacokinetic pilot study. AIDS 2000; 14(9): F103–10
Veldkamp AI, van Heeswijk RPG, Mulder JW, et al. Steady-state pharmacokinetics of twice-daily dosing of saquinavir plus ritonavir in HIV-1-infected individuals. J AIDS 2001; 27: 344–9
Acosta EP, Zorrilla C, Bardeguez A, et al. pharmacokinetics (PK) of saquinavir/ritonavir (SQV/RTV) in HIV-infected pregnant women [abstract no. A-1405]. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2002 Sep 27–30; San Diego, 22
Landman R, Peytavin G, Lamotte C, et al. Saquinavir (SQV) plasma and intracellular concentrations in a once daily dosing combination fortovase (SQV-SGC) —low dose ritonavir (RTV) in a prospective study (IMEA 015) in HIV-infected patients (pts) [abstract no. 179]. Ist international AIDS Society Conference on HIV Pathogenesis and Treatment; 2001 Jul 8–11; Buenos Aires
López-Cortés LF, Ruiz-Valderas R, Viciana P, et al. Once-daily saquinavir-sgc plus low-dose ritonavir (1200/100 mg) in combination with efavirenz; pharmacokinetics and efficacy in HIV-infected patients with prior antiretroviral therapy [letter]. J. Acquir. Immune. Defic. Syndr. 2003 Feb 1; 32(2): 240–2
Mallolas J, Blanco J, Sarasa M, et al. Intensification therapy with saquinavir soft gel (SSG)/ritonavir (RIV) QD in patients failing on a saquinavir hard gel (SHG) containing HAART [abstract no. 675]. 1st International Aids Society Conference; 2001 Jul 8–11; Buenos Aires, 282
Hellinger J, Morris AB, Piscitelli S, et al. Pilot study of saquinavir-SGC (Fortovase, AQV) 1000 mg twice daily and lopinavir/ritonavir (Kaletra, LPV/r) in protease inhibitor-experienced HIV+ individuals: dose escalation and combined normalized inhibitory quotient (cNIQ) [poster no. 451-W]. 9th Conference on Retroviruses and Opportunistic Infections; 2002 Feb 24–28; Seattle
Smith GHR, Klein M, Leblanc R, et al. Salvage therapy for HIV-1 infection with lopinavir/ritonavir (LOP/RIT), saquinavir-SGC (SQR), and nucleoside analogues in patients having failed all three antiretroviral classes [abstract no. 212]. Can J Infect Dis 2001; 12 Suppl. B: 29B
Back D, Gatti G, Fletcher C, et al. Therapeutic drug monitoring in HIV infection: current status and future directions. AIDS 2002; 16 Suppl. 1: S5–37
Stephan C, von Hentig N, Moesch M, et al. Saquinavir plasma exposure is not decreased in lopinavir/ritonavir co-administration compared to standard ritonavir booster by AUC in steady state condition [poster]. 6th International Congress on Drug Therapy in HIV Infection; 2002 Nov 17–21; Glasgow
SQV/LPV/r has a favourable PK profile making it a logical choice for double boosting. Reports from the 6th International Congress on Drug Therapy in HIV Infection; 2002 Nov 17–21; Glasgow. Roche, 2002. (Data on file)
Ribera E, Lopez RM, Pou L, et al. Pharmacokinetics of a double boosting regimen of lopinavir, plus minidose ritonavir, plus saquinavir in HIV-infected adults [poster no. TuPeB4545]. XIV International AIDS Conference; 2002 Jul 7–12; Barcelona
Corbett AH, Eron JJ, Diebold M, et al. A triple protease inhibitor [PI] salvage regimen of amprenavir [APV] + saquinavir [SQV] + minidose ritonavir [r]: steady state [SS] pharmacokinetics [PK] and initial RNA and CD4 response [abstract no. TuPeB4464]. XIV International AIDS Conference; 2002 Jul 7–12; Barcelona, 383
Corbett AH, Eron JJ, Diebold M, et al. Short-term safety and efficacy of an amprenavir (APV)/saquinavir (SQV)/ritonavir (RTV) salvage regimen [abstract no. 450]. 40th Annual Meeting of the Infectious Diseases Society of America; 2002 Oct 24–27; Chicago
Ribera E, Azuaje C, Montero F, et al. Saquinavir, ritonavir, didanosine, and lamivudine in a once daily regimen for HIV infection in patients with rifampin-containing antituberculosis treatment [poster no. ThPeB7280]. XIV International AIDS Conference; 2002 Jul 7–12; Barcelona
Veldkamp AI, Hoetlmans RMW, Beijnen JH, et al. Ritonavir enables combined therapy with rifampin and saquinavir. Clin Infect Dis 1999; 29: 1586
Gallicano K, Khaliq Y, Carignan G, et al. A pharmacokinetic study of intermittent rifabutin dosing with a combination of ritonavir and saquinavir in patients infected with human immunodeficiency virus. Clin Pharmacol Ther 2001 Aug; 70(2): 149–58
Boyd M, Ruxrungtham K, Zhang X, et al. Enfuvirtide —investigations on the drug interaction potential in HIV-infected patients [poster no. 541]. 10th Conference on Retroviruses and Opportunistic Infections; 2003 Feb 10–14; Boston
Zorrilla CD, Van Dyke R, Bardeguez A, et al. Clinical response, safety, and tolerability to saquinavir-SCG with low-dose ritonavir boosting in combination with zidovudine and lamivudine in HIV-1 infected pregnant women: preliminary results of PACTG 386 [abstract no. 887 and poster no. P-23]. 10th Conference on Retroviruses and Opportunistic Infections; 2003 Feb 10–14; Boston
Gerstoft J, Dragsted UB, Cahn P, et al. Final analysis of a randomised trial to evaluate safety and efficacy of indinavir/ ritonavir versus saquinavir/ritonavir in adult HIV-1 infection: the MaxCmin1 trial [abstract no. H-172 and poster]. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2002 Sep 27–30; San Diego
Saquinavir/r versus indinavir/r: final data from the MaxCmin1 trial. Reports from the 6th International Congress on Drug Therapy in HIV Infection; 2002 Nov 17–21; Glasgow. Roche, 2002. (Data on file)
Hoffmann-La Roche Inc. Head-to-head HIV study showed significant benefit for boosted saquinavir over boosted indinavir [media release] [online]. Available from URL: http://www.rocheusa.com [Accessed 2002 Jul 18]
Dragsted UB, Gerstoft J, Youle M, et al. The interim analysis of a phase IV, randomised, open-label, multi-centre trial to evaluate safety and efficacy of lopinavir/ritonavir (400/100 mg bid) versus saquinavir/ritonavir (1000/100 mg bid) in adult HIV-1 infection: the MaxCmin2 trial [poster no. PL14.5]. 6th International Congress on Drug Therapy in HIV Infection; 2002 Nov 17–21; Glasgow
Saquinavir/r and lopinavir/r go head-to-head: 24-week data from the MaxCmin2 trial. Reports from the 6th International Congress on Drug Therapy in HIV INfection; 2002 Nov 17–21; Glasgow. Roche, 2002. (Data on file)
Hoffmann-La Roche Inc. Use of boosted saquinavir or Kaletra(TM) results in HIV suppression in most patients at 24 weeks [media release] [online]. Available from URL: http://www.rocheusa.com [Accessed 2002 Dec 4]
Piketty C, Race E, Castiel P, et al. Phenotypic resistance to protease inhibitors in patients who fail on highly active antiretroviral therapy predicts the outcome at 48 weeks of a five-drug combination including ritonavir, saquinavir and efavirenz [letter]. AIDS 2000; 14(5): 626–8
Piketty C, Trylesinski A, Hill A, et al. Plasma drug levels and resistance as predictors of efficacy for the combination including saquinavir, ritonavir and efavirenz [abstract]. Antiviral Ther 2001; 6 Suppl. 1: 89
Chaillou S, Durant J,Clevenbergh P, et al. Long term efficacy of saquinavir and ritonavir low dosage (baby-dose) in HIV patients failing combination therapy: correlation of response to treatment with genotypic mutations and pharmacologic data [abstract no. 565]. 7th European Conference on Clinical Aspects and Treatment of HIV-Infection; 1999 Oct 23–27; Lisbon
Montaner JSG, Saag MS, Barylski C, et al. FOCUS study: saquinavir QD regimen versus efavirenz QD regimen 48 week analysis in HIV infected patients [abstract no. H-167 and poster]. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; 2002 Sep 27–30; San Diego
Montaner JSG, Saag M, Barylski C, et al. FOCUS study: saquinavir QD regimen versus efavirenz QD regimen 48 week analysis in HIV infected patients [abstract no. 1-670 plus oral presentation]. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; 2001 Sep 22–25; Chicago
Cardiello P, Srasuebkul P, Hassink E, et al. The efficacy, safety, and immunological changes of qd saquinavir-soft gel capsules 1600 mg/ritonavir 100 mg plus dual nucleosides in patients who had an undetectable viral load after 3 years of treatment [poster no. 549-T]. 9th Conference on Retroviruses and Opportunistic Infections; 2002 Feb 24–28; Seattle
Hoffmann-La Roche Inc. Fortovase with Kaletra shows encouraging results among protease inhibitor-experienced HIV patients in initial study — a second study evaluates viral and immune response of switch to once-daily fortovase with ritonavir from twice-daily Fortovase [media release]. 2002 Mar 1
Mars M, Trylesinski A, Loi S, et al. Fortovase-ritonavir (FTV/ R) QD used after a first protease inhibitor (PI) therapy in HIV infected patients (pts) with virologic failure [abstract no. 676]. 1st International Aids Society Conference; 2001 Jul 8–11; Buenos Aires, 282
Wright DP, Pithey C. Virologic improvement in patients receiving once-daily saquinavir/min-dose ritonavir (SQV/r) therapy in clinical practice [abstract no. 328]. Clin Infect Dis 2000 Jul; 31(1): 269
Baril JG, Trottier B, LeBlanc R, et al. Efficacy of switching virologically suppressed patients from a protease inhibitor (PI) based therapy to a saquinavir (SQV) and low dose ritonavir (RTV) once daily dosing regimen with therapeutic drug monitoring (TDM) [poster]. 6th International Congress on Drug Therapy in HIV Infection; 2002 Nov 17–21; Glasgow
Baril JG, Trottier B, LeBlanc R, et al. Efficacy of once daily saquinavir (SQV) and low dose ritonavir (RTV) containing salvage therapy in heavily pretreated patients who were not virologically suppressed [poster]. 6th International Congress on Drug Therapy in HIV Infection; 2002 Nov 17–21; Glasgow
Staszewski S, Dauer B, Stephan C, et al. Switch to a simple boosted double protease inhibitor regimen of lopinavir/r and saquinavir without reverse transcriptase inhibitors after multiple therapy failures (poster TuPeB4427). Roche, 2002. (Data on file)
Ruiz L, Ribera E, Bonjochl A, et al. Virological and immunological benefit of a salvage therapy that includes Kaletra plus Fortovase preceded or not by antiretroviral therapy interruption (TI) in advanced HIV-infected patients (6-month follow-up) [poster no. 421-W]. 9th Conference on Retroviruses and Opportunistic Infections; 2002 Feb 24–28; Seattle
Zala C, Patterson P, Coll P, et al. Early antiviral response and safety of a dual boosted protease inhibitors regimen including lopinavir/r (LPV) plus amprenavir (AMP) or Fortovase (FTV) in heavily pretreated HIV infected patients. Roche, 2002. (Data on file)
Hoffmann-La Roche Inc. Four studies examine strategy of combining saquinavir with Kaletra (TM) in treatment-experienced HIV patients [media release] [online]. Available from URL: http://www.rocheusa.com [Accessed 2002 Jul 15]
Khanlou H, Graham E, Brill M, et al. Drug interaction between amprenavir and lopinavir/ritonavir in salvage therapy [letter]. AIDS 2002; 16: 797–812
Mauss S, Schmutz G, Kuschak D. Unfavourable interaction of amprenavir and lopinavir in combination with ritonavir [letter]? AIDS 2002; 16(2): 296–7
Smith GHR, Klein MB, Murphy T, et al. Double, boosted salvage therapy with lopinavir/ritonavir and saquinavir-sgc in HIV-1 infected patients having failed 3 classes of antire-trovirals. Roche, 2001. (Data on file)
Stephan C, Lutz T, Haberl A, et al. Lopinavir/r —saquinavir without RTI in pre-treated patients with NUC associated resistance or toxicity [poster no. LB/P8]. 8th European Conference on Clinical Aspects and treatment of HIV-Infection; 2001 Oct 28–31; Athens
Gill MJ. Safety profile of soft gelatin formulation of saquinavir in combination with nucleosides in a broad patient population. NV15182 Study Team [letter]. AIDS 1998 Jul 30; 12(11): 1400–2
Mitsuyasu RT, Skolnik PR, Cohen SR, et al. Activity of the soft gelatin formulation of saquinavir in combination therapy in antiretroviral-naive patients. NV15355 Study Team. AIDS 1998 Jul 30; 12(11): F103–9
O’Brien WA, Acosta E, Felizarta F, et al. Switch of saquinavir 400 mg/ritonavir 400 mg to saquinavir 1000 mg/ritonavir 100 mg during BID four drug antiretroviral therapy [poster no. WeOrB1263]. XIV International World AIDS Conference; 2002 Jul 7–12; Barcelona
Srasuebkul P, Cardiello P, Hassink E, et al. Gastrointestinal toxicity and triglyceride levels when switching from a twice daily to a once daily saquinavir soft gel regimen. Roche, 2002. (Data on file)
Walmsley S, Montaner J, Hill A, et al. NRTI treatment as a risk factor for hyperlipidemia: results from the FOCUS trial [poster]. 4th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV; 2002 Sep 22–25; San Diego
Hoffmann-La Roche. Roche-HIV.com/Newsroor/Latest News [online]. Available from URL: http://wwwURL.roche-hiv.com [Accessed 2003 Feb 24]
British National Formulary. Saquinavir [online]. Available from URL: http://www.bnf.org [Accessed 2003 Feb 7]
Blaschke T, Flexner C, Sheiner L, et al. Effect of simultaneous or staggered dosing of saquinavir (SQV), ritonavir (RTV), and nelfinavir (NFV) on pharmacokinetic (PK) interactions [abstract no. 76]. 7th Conference on Retroviruses and Opportunistic Infections; 2000 Jan 30–Feb 4; San Francisco
Gulick RM, Mellors JW, Havlir D, et al. 3-Year suppression of HIV viremia with indinavir, zidovudine, and lamivudine. Ann Intern Med 2000 Jul 4; 133(1): 35–9
Gulick RM, Mellors JW, Havlir D, et al. Simultaneous vs sequential initiation of therapy with indinavir, zidovudine, and lamivudine for HIV-1 infection: 100-week follow-up. JAMA 1998 Jul 1; 280(1): 35–41
Cameron DW, Angel JB, Ryan J, et al. Durability of ritonavir plus saquinavir dual protease inhibitor therapy in HIV infection: 5-year follow-up [abstract no. 550-T]. 9th Conference on Retroviruses and Opportunistic Infections; 2002 Feb 24–28; Seattle
Carpenter CCJ, Cooper DA, Fischl MA, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society —USA Panel. 2000 Jan 19; 283: 381–90
Gazzard B, Moyle G. 1998 revision to the British HIV Association guidelines for antiretroviral treatment of HIV seropositive individuals. BHIVA Guidelines Writing Committee. Lancet 1998 Jul 25; 352: 314–6
European guidelines for the clinical management and treatment of HIV infected adults in Europe 2001 [online]. Available from URL: http://www.iapac.org [Accessed 2003 Feb 20]
International Association of Physicians in AIDS Care (IAPAC). Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents [online]. Available from URL: http://www.iapac.org [Accessed 2003 Feb 20]
World Health Organization (WHO). Scaling up antiretroviral therapy in resource-limited settings: guidelines for a public health approach [online]. Available from URL: http://www.who.int/hiv/pub [Accessed 2003 Feb 20]
Abbott Laboratories. Kaletra prescribing information. Chicago (IL): Abbott Laboratories, 2002
Cvetkovic RS, Goa KL. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs 2003; 63(8): 769–805
Moyle G. Overcoming obstacles to the success of protease inhibitors in highly active antiretroviral therapy regimens. AIDS Patients Care STDS 2002 Dec; 16(12): 585–97
Haas DW, Zala C, Schrader S, et al. Atazanavir plus saquinavir once daily favorably affects total cholesterol (TC), fasting triglyceride (TG), and fasting LDL cholesterol (LDL) profiles in patients failing prior therapy (trial AI424-009, week 48) [abstract no. 42]. 9th Conference for Retroviruses and Opportunistic Infections; 2002 Feb 24–28; Seattle
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: R.C. Brundage, Graduate Program in Experimental and Clinical Pharmacology, University of Minnesota, Minneapolis, Minnesota, USA; P.J. Carey, Sunderland Royal Infirmary, Sunderland, England; B. Conway, Department of Pharmacology and Therapeutics, University of British Columbia, Vancouver, British Columbia, Canada; V. Joly, Hôpital Bichat-Claude Bernard and Xavier-Bichat Medical School, Paris, France; A.D.M. Kashuba, School of Pharmacy and UNC Center for AIDS Research, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA; J. Mallolas, Department of Infectious Diseases, Institut D’Investigacions Biomediques August Pi I Sunyer (IDIBAPS) Fundacio Clinic, Barcelona, Spain; S.R. Penzak, Clinical Center Pharmacy Department, National Institutes of Health, Bethesda, Maryland, USA; R.P.G. van Heeswijk, Division of Infectious Diseases, The Ottawa Hospital, Ottawa, Ontario, Canada; C. Zala, Clinical Research, Fundacion Huesped, Buenos Aires, Argentina.
Data Selection
Sources: Medical literature published in any language since 1980 on saquinavir, identified using Medline and EMBASE, supplemented by AdisBase (a proprietary database of Adis International). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: Medline search terms were ‘saquinavir and boosted’ or ‘saquinavir and pharmacoenhancement’ or ‘saquinavir and protease inhibitors’ or ‘saquinavir and HIV infections’. EMBASE search terms were ‘saquinavir and boosted’ or ‘saquinavir and pharmacoenhancement’ or ‘saquinavir and protease inhibitors’ or ‘saquinavir and HIV infections’. AdisBase search terms were ‘saquinavir and boosted’ or ‘saquinavir and protease-inhibitors’ or ‘saquinavir and HIV-infections’. Searches were last updated 15 April 2003.
Selection: Studies in patients with HIV infection who received boosted saquinavir regimens. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Saquinavir, boosted regimens, pharmacoenhancement, pharmacodynamics, pharmacokinetics, pharmacokinetic enhancement, drug interactions, therapeutic use, tolerability, HIV infection.
Rights and permissions
About this article
Cite this article
Plosker, G.L., Scott, L.J. Saquinavir. Drugs 63, 1299–1324 (2003). https://doi.org/10.2165/00003495-200363120-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200363120-00007