Abstract
Background: There are over 40 000 ischaemic strokes annually in Canada, which result in significant morbidity, mortality and burden to the healthcare system. A recent, large clinical trial has evaluated tissue plasminogen activator (t-PA) intravenously for the treatment of acute ischaemic stroke with promising outcomes but with an increased risk of symptomatic intracranial haemorrhage.
Objective: To compare clinical and economic outcomes of intravenous t-PA therapy (0.9 mg/kg, to a maximum of 90mg, initiated within 3 hours of stroke onset) versus no t-PA for acute ischaemic stroke based on the outcomes achieved in the National Institute of Neurological Disorders and Stroke (NINDS) trial.
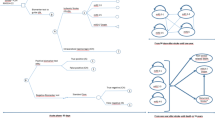
Design: A Markov model depicting the natural lifetime course after an initial acute ischaemic stroke. On the basis of this model, a simulated trial compared no t-PA with t-PA.
Patients: A hypothetical cohort of 1000 patients with acute ischaemic stroke.
Study perspective: Canadian healthcare system.
Outcome measures: Total acute stroke and post-stroke treatment costs and cumulative quality-adjusted life-years (QALYs).
Results: For a hypothetical cohort of 1000 patients, the estimated lifetime stroke costs were 103 100 000 Canadian dollars ($Can) [1999 values) in the t-PA arm ($Can103 100 per patient) compared with $Can106 900 000 in the no t-PA arm ($Can106 900 per patient), yielding a lifetime cost difference of $Can3 800 000 in favour of t-PA versus no t-PA ($Can3800 per patient). In the hypothetical cohort, t-PA treatment resulted in 13 130 QALYs versus 9670 QALYs with no t-PA treatment. This translated into a net benefit of 3460 additional QALYs per 1000 patients (3.46 QALYs per patient). No treatment, outcome or economic variables influenced the model outcome.
Conclusion: From the standpoint of cost effectiveness, treatment of acute ischaemic stroke with intravenous t-PA is an economically attractive strategy.






Similar content being viewed by others
References
Fisk B, Hebden T, MacGregor L, et al. Heart disease and stroke in British Columbia 1998. Vancouver (BC): Heart and Stroke Foundation of B.C. and Yukon, 1998
Gordon M. Monograph series on aging-related diseases: III. Stroke (cerebrovascular disease). Chronic Dis Can 1993; 14: 64–89
Wittkowsky AK. The stroke pharmacopeia: current medical therapies. Pharmacotherapy 1998; 18: 94S–100S
Health Canada. Heart disease and stroke in Canada 1997. Health Canada [online]. Available from: URL: http://www.hcsc. gc.ca/hpb/lcdc/bcrdd/hdsc97/tab03_e.html [Accessed 1999 Sep 21]
Alexandrov AV, Smurawska LT, Bartle W, et al. Cost considerations in the pharmacological prevention and treatment of stroke. Pharmacoeconomics 1997; 11: 408–15
Smurawska LT, Alexander AV, Bladin CF, et al. Cost of acute stroke care in Toronto, Canada. Stroke 1994; 25: 1628–31
National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–7
Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the European Cooperative Acute Stroke Study (ECASS). JAMA 1995; 274: 1017–25
Hacke W, Kaste M, Fieschi C, et al., Second European-Australasian Acute Stroke Study Investigators. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet 1998; 352: 1245–51
Adams HP, Brott TG, Furlan AJ, et al. Member’s guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke. A statement for healthcare professionals from a special writing group of the stroke council, American Heart Association. Circulation 1996; 94: 1167–74
Fagan SC, Morgenstern LB, Petitta A, et al. Cost-effectiveness of tissue plasminogen activator for acute ischemic stroke. Neurology 1998; 59: 883–900
Briggs A, Sculpher M. An introduction to Markov modeling for economic evaluation. Pharmacoeconomics 1998; 13: 397–409
van Sweiten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988; 19: 604–7
Solomon NA, Glick HA, Russo CH, et al. Patient preferences for stroke outcomes. Stroke 1994; 25: 1721–5
Canadian Coordinating Office of Health Technology Assessment. Guidelines for the economic evaluation of pharmaceuticals: Canada. 2nd ed. Ottawa (ON): Canadian Coordinating Office of Health Technology Assessment (CCOHTA), 1997
Statistics Canada. Population. Statistics Canada [online]. Available from: URL: http://www.statcan.ca/english/Pgdb/People/ Population/demo02.htm [Accessed 1999 Sep 22]
Statistics Canada. National population health survey overview 1996–97. Ottawa (ON): Minister of Industry, 1998
British Columbia Medical Association. Guide to fees. Vancouver (BC): British Columbia Medical Association, 1998
Jacobs P, Hall EM, Bachnysky J. An Alberta standard cost list for health economics evaluations. Ottawa (ON): Canadian Coordinating Office of Health Technology Assessment (CCOHTA), 1997
Chan B, Hayes B. Cost of stroke in Ontario, 1994/95. CMAJ 1998; 159: S2–S8
Hodgson C. Prevalence and disabilities of community-living seniors who report the effects of stroke. CMAJ 1998; 159: S9–S14
Heart and Stroke Foundation of Ontario. Heart and Stroke Foundation of Ontario [online]. Available from: URL: http://www.hsf.on.ca/facts_stroke.htm [Accessed 1999 Sep 23]
Samsa G, Reutter R, Parmiigiani G, et al. Performing cost-effectiveness analysis by integrating randomized trial data with a comprehensive decision model: application to treatment of acute ischemic stroke. J Clin Epidemiol 1999; 52: 259–71
Oh P, Pritchard L. Thrombolytic therapy in stroke [abstract]. Can J Clin Pharmacol 1997; 4: 50
Nadareishvili Z, Oh P, Smurawska LT, et al. Cost-effectiveness of tissue plasminogen activator for acute ischemic stroke [letter]. Neurology 1999; 52: 895
Tran C, Nadareishvili Z, Oh P. Decreasing costs of stroke hospitalization in Toronto. Stroke 1999; 30: 185–6
Wardlaw JM, del Zoppo G, Yamaguchi T. Thrombolysis for acute ischaemic stroke [review]. Cochrane Database Syst Rev 2000; 2: CD000213
Kwiatkowski TG, Libman RB, Frankel M, et al. Effects of tissue plasminogen activator for acute ischemic stroke at one year. N Engl J Med 1999; 340: 1781–7
Grond M, Stenzel C, Schmulling S, et al. Early intravenous thrombolysis for acute ischemic stroke in a community-based approach. Stroke 1998; 29: 1544–9
Wang DZ, Rose JA, Honings DS, et al. Treating acute stroke patients with intravenous tPA. Stroke 2000; 31: 77–8131
Albers GW, Bates VE, Clark WM, et al. Intravenous tissue-type plasminogen activator for treatment of acute stroke (STARS study). JAMA 2000; 283: 1145–50
Chiu D, Kreiger D, Villar-Cordova C, et al. Intravenous tissue plasminogen activator for acute ischemic stroke: feasibility, safety, and efficacy in the first year of clinical practice. Stroke 1998; 29: 18–22
Katzan IL, Furlan AJ, Lloyd LE, et al. Use of tissue-type plasminogen activator for acute ischemic stroke. JAMA 2000; 283: 1151–8
Acknowledgements
This study was funded by the B.C. and Yukon Heart and Stroke Foundation. Dr Teal has functioned as a consultant to Hoffman LaRoche and Dr Fagan is on the Speaker’s Bureau and has been a consultant for Genentech, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sinclair, S.E., Frighetto, L., Loewen, P.S. et al. Cost-Utility Analysis of Tissue Plasminogen Activator Therapy for Acute Ischaemic Stroke. Pharmacoeconomics 19, 927–936 (2001). https://doi.org/10.2165/00019053-200119090-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00019053-200119090-00004