Abstract
Interferon-α (IFNα) is a pleiotropic cytokine with direct and indirect antitumour effects. These include prolongation of the cell cycle time of malignant cells, inhibition of biosynthetic enzymes and apoptosis, interaction with other cytokines, and immunomodulatory and antiangiogenic effects. The first clinical trials in solid tumours used crude preparations of natural IFNα and demonstrated that tumour regressions in solid tumours and haematological malignancies were possible. Since the advent of genetic engineering technology, recombinant (r) IFNα has been widely evaluated in solid tumours. This review discusses the use and potential of rIFNα in solid tumours; the first part focuses on malignant melanoma and metastatic renal cell carcinoma (RCC).
In the adjuvant treatment of malignant melanoma, rIFNα has been tested in randomised trials in more than 6000 patients. High-dosage IFNα (≥10MU) prolongs disease-free survival (DFS) but not overall survival (OS). Low-dosage IFNα (≤3MU) has not been shown to prolong DFS or OS, and current data do not support its use outside clinical trials. The latest United Kingdom Co-ordinating Committee on Cancer Research meta-analysis of ten randomised trials that used adjuvant rIFNα has shown that there is a benefit in DFS but not OS. No conclusions can be reached for intermediate-dosage IFNα (5 to 10MU) until the mature results of the European Organization for Research and Treatment of Cancer (EORTC) study 18952 are available. In RCC, current evidence does not support the use of adjuvant IFNα.
In metastatic malignant melanoma and RCC, reported response rates to rIFNα are approximately 15%. In a minority of responding patients, however, these responses can be long-standing. In metastatic malignant melanoma, IFNα combined with other cytotoxic agents with or without interleukin-2 has achieved high response rates but has not improved survival. In metastatic RCC, intermediate dosages of rIFNα should be used and therapy should probably be prolonged (≥12 months); response depends on prognostic factors such as good performance status, whereas survival is affected by factors such as low tumour burden. Nephrectomy should therefore be considered in patients with good performance status prior to IFNα immunotherapy in advanced RCC, even in patients with metastatic disease.
The toxicity of high-dosage IFNα and the lack of definite benefit on OS with high- or low-dosage IFNα do not support its use outside clinical trials. Data from the ongoing US Intergroup studies, the ongoing EORTC 18991 study (long-term therapy with pegylated IFNα) and mature data from EORTC 18952 (intermediatedosage IFNα) will help establish the role of IFNα as adjuvant therapy in malignant melanoma.
Similar content being viewed by others
Isaacs and Lindenmann discovered interferon in 1957 while searching for a substance that blocked viral infection of cells.[1] The medium from cultures of chicken cells, infected with influenza virus, was added to other cultures of chicken cells. This medium prevented the infection of the second cultures by a different virus. The interfering substance was designated interferon. Nagano and Kojima also independently discovered this soluble antiviral protein.[2]
Interferons are a family of proteins that exhibit antiviral activity.[3] Human interferons were initially categorised into leucocyte, fibroblast and immune interferons on the basis of their cellular origin.[3,4] Interferons are divided into two types based on their ability to bind a common receptor. Type I interferons are acid stable and are the ligands for the type I interferon receptor; they include leucocyte or α interferon (IFNα), fibroblast or β interferon (IFNβ), τ interferon and ω interferon.[5] Type II interferon is acid labile, binds the type II interferon receptor, and is represented by immune or γ interferon (IFNγ).[5] The α interferons are glycosylated polypeptides of varying molecular weight that are produced in response to enveloped viruses, virus-infected cells, bacteria and tumour cells.[6] Fibroblasts, epithelial cells and macrophages produce IFNβ following exposure to micro-organisms.[7] IFNγ is produced by lymphocytes after antigenic or mitogenic stimulation.[7] Table I summarises the classification of interferons and their properties.
1. Biology of Interferon-α
1.1 Natural Leucocyte Interferon
Natural leucocyte interferon can be produced in vitro after treating leucocytes with a virus such as Sendai or Newcastle disease virus.[4] Cantell and colleagues[10] were the first to produce partially purified IFNα. In 1979, high performance liquid chromatography was used to isolate and characterise natural leucocyte interferon; this was found to consist of at least ten species that were designated α-1, α-2, β-1 to 3, γ-1 to 5 and δ.[4,11] The same Greek letters were used a year later to formally classify interferons as α, β and γ, corresponding to the previous names of leucocyte, fibroblast and immune interferon.[12] The different molecular forms of natural leucocyte interferon were shown to exhibit varying cytostatic and immunomodulatory effects on mammalian cells.[13]
In vivo, the main producers of IFNα are the natural interferonproducing cells, specialised leucocytes producing huge amounts of IFNα in response to enveloped viruses, bacteria and tumour cells.[14] Although leucocytes are the prototypic cells producing IFNα, nearly all cells in the body can produce it.[8]
1.2 Genes, Proteins and Receptors
The genes that encode IFNα have been cloned.[15,16] To date, 14 human IFNα genes have been discovered,[4] which encode for 12 distinct IFNα proteins.[3] The cloning of a gene (IFNA2) for the human IFNα-2 protein[17,18] and the advent of recombinant genetic engineering allowed the production of sufficient IFNα-2 for clinical use. Recombinant human IFNα-2 is available in two forms, rhIFNα-2a and rhIFNα-2b.[7] In some countries rhIFNα-2c is licensed. Recombinant IFNα-2a, -2b and -2c differ in one to two amino acid positions.[9] Although rIFNα2c has been tested in clinical trials, the antitumour activities of rhIFNα-2a, -2b and -2c in vivo have not been compared with each other in randomised studies.
Interferons bind specific high-affinity receptors on the cell surface.[7] The IFNα receptor (IFNα-R) is made up of at least two polypeptide chains, IFNα-R1 and IFNα-R2 (figure 1).[5] IFNα-R1 is involved in signal transduction[19] and IFNα-R2 is the ligand-binding chain that also plays a role in signal transduction.[19] IFNα-R2 exists in three isoforms,[20] of which IFNα-R2c is the functional receptor.[21] Ligation of the receptor by IFNα produces oligomerisation of the receptor chains followed by a series of phosphorylations and initiation of a signal transduction pathway. This results in the phosphorylation of STAT (signal transducers and activators of transcription) proteins, which translocate to the nucleus as a trimeric complex, ISGF-3 (interferon-stimulated gene factor-3). ISGF-3 activates transcription of interferon-stimulated genes, with many biological effects.[21]
Signal transduction pathway for interferon-α (IFNα) signalling: (i) receptor (IFNαR) ligation and oligomerisation; (ii) transphosphorylation of Janus kinases(JaKs; JaK 1 and TyK 2); (iii) phosphorylation of the cytoplasmic tails of receptors; (iv) docking of STAT (signal transducers and activators of transcription) proteins on the cytoplasmic tails of the receptor and phosphorylation (-P) by JaKs; and (v) STAT dimers heterodimerise with p48 to form IFNα-stimulated gene factor-3 (ISGF-3), which translocates to the nucleus to activate transcription of IFNα-sensitive genes by binding to IFNα-stimulated response elements (ISRE) [reproduced from Pestka,[20] with permission].
1.3 Mechanisms of Action and Antitumour Effects
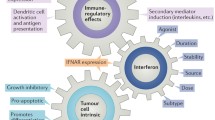
IFNα is a pleiotropic cytokine[22] with antiviral and antitumour effects,[20] and immunomodulatory[7] and antiangiogenic activity (table II).[6,23] The complexity of its effect on cellular function is illustrated by the fact that IFNα can induce the expression of more than 300 genes.[24] The antitumour action of IFNα is mediated by the following effects.
-
(i)
The antiviral effect of IFNα is shown by its ability to limit virally induced malignancies such as hepatitis virus-induced liver cancers and herpes simplex virus-associated Kaposi’s sarcoma.[25]
-
(ii)
IFNα has a number of direct antitumour effects, which include prolongation of the cell cycle of neoplastic cells,[7] i.e. a cytostatic mechanism. This is thought to be achieved by modulation of 2′,5′-oligoadenylate synthetase activity[27] or modulation of cellular oncogenes.[29] IFNα also inhibits ornithine decarboxylase,[28] an important biosynthetic enzyme, and thus may lead to depletion of metabolites required for tumour growth. IFNα may also interact synergistically with IFNγ to promote cell lysis.[30] Relatively recently, it has been demonstrated that IFNα has proapoptotic effects in human tumour cell lines, squamous cell cancers and basal cell carcinoma.[26]
-
(iii)
IFNα exerts indirect antitumour effects. These include upregulation of class I major histocompatibility complex (MHC) and other membrane antigens on malignant cells (e.g. melanoma tumour-specific antigens),[31] resulting in more efficient recognition/lysis of cancer cells by immune effector cells.[32] Production of antibodies to tumour cells is enhanced, resulting in increased complement-mediated and antibody-dependent cell-mediated cytotoxicity of tumour cells.[32] This may explain the augmented cytotoxicity of macrophages, natural killer (NK) cells and T cells.[33,34]
-
(iv)
The other important indirect mode of action of IFNα is by inhibition of angiogenesis.[35] This was observed initially in mice.[36] It was subsequently observed that recombinant IFNα therapy improved Kaposi’s sarcoma, the vascular tumour associated with HIV infection,[37] and shown that IFNα produces regression of haemangiomas, the most common benign tumour of infancy.[38–40]
1.4 Effect on Function of Cells of the Immune System
IFNα has a pivotal role in promoting T helper 1 (Th1) differentiation of naive T helper cells (Th0). From the clinical viewpoint, Th1 responses, characterised by production of interleukin (IL)-2, IFNα and tumour necrosis factor (TNF), are important because they confer protective immunity to pathogens and tumours. Th2 responses, characterised by production of IL-4, IL-5, IL-6, IL-10 and IL-13,[41] are associated with hypersensitivity reactions and autoimmune disease.[42] IFNα makes T cells more responsive to IL-12 (figure 2), thus promoting a Th1 immune response.[42] In addition to this effect, IFNα induces the generation and survival of CD8+ memory cells and is therefore important for cytotoxic T cell responses.[42] IFNα has a stimulatory effect on macrophages[8] and enhances the cytotoxic activity of NK cells.[43]
Effect of interferon-α (IFNα) in promoting T helper 1 (Th1) differentiation. Antigen (Ag) presentation by major histocompatibility complex class II (MHC II) on an antigen-presenting cell (APC) to the T cell receptor (TCR) on a naïve T cell promotes IFNα production by the APC (i). IFNα binds to its receptor (IFNαR) on the T cell (ii) and initiates the signal transduction pathway (figure 1), resulting in transcription of IFNα-stimulated genes (iii). One of the effects is upregulation of the β2 subunit of the interleukin-12 receptor (IL-12R) (iv). The high affinity receptor (IL-12Rβ1 + IL-12Rβ2) makes the T cell more responsive to IL-12 produced by the APC (v). Binding of IL-12 to its receptor (vi) activates STAT (signal transducers and activators of transcription) protein 4 (vii), which is important for IFNγ production (viii). IFNγ in turn promotes a Th1 immune response (ix) [reproduced from Tompkins,[42] with permission].
2. History of Interferon-α in the Treatment of Solid Tumours
The initial clinical trials used crude preparations, containing <1% IFNα, isolated from buffy coats.[3] The earliest use of IFNα in human cancer was by Strander, who had treated patients with osteosarcoma since 1971. In his series, 64% of interferontreated patients were free of metastases at 2.5 years after surgery compared with 30% in a control group from other Swedish hospitals. Overall survival (OS) for Strander’ s patients was also improved at 73%, compared with 35% in controls, at 2.5 years.[44–46] After this, tumour regressions were reported in cervical cancer,[47] non-Hodgkin’s lymphoma[48] and breast cancer, myeloma, lymphoma and malignant melanoma.[49–51] In 1981, interferon was used in the neoadjuvant setting in patients with cervical cancer, and tumour regressions were again seen.[52] In 1982, a phase II study of the American Cancer Society reported a 22% response rate in breast cancer patients.[53] The numbers of patients treated in these trials were small; however, leucocyte interferon was shown to have antitumour activity. These trials were therefore very important because they provided the impetus for the development of rIFNα to provide sufficient quantities for clinical use.
The availability of large amounts of rIFNα from 1981 onwards allowed numerous phase I, II and III trials to be conducted,[54] in which its efficacy, tolerability and effect on survival in patients with solid tumours and haematological malignancies were tested. The US Food and Drug Administration (FDA) approved rIFNα for clinical use in hairy cell leukaemia in 1986.[22] Since then, approval has been given for use in other malignancies, including malignant melanoma, renal cell carcinoma (RCC) and Kaposi’s sarcoma.[22] Some tumours such as superficial bladder cancer are characterised by very high response rates; in malignant melanoma and RCC the response rate is low,[55] but these responses are extremely important because some of them are long-lasting. In addition, IFNα has been used to treat head and neck cancers, gastrointestinal tract and neuroendocrine tumours, and lung, breast, ovarian and cervical cancers (reviewed by Jonasch and Haluska,[8] John and Foon,[56] and Borden et al.[57]).
3. Malignant Melanoma
3.1 Adjuvant Therapy
Malignant melanoma accounts for 2% of all cases of cancer and 1% of all cancer deaths in the UK.[58] However, its incidence is rising and in some areas of Australia and New Zealand 1 in 30 people will develop melanoma, while in the US the life-time risk of developing melanoma has risen by more than 2-fold in the last 20 years.[59] The 5-year survival rate for patients with American Joint Committee on Classification (AJCC) stage I disease (primary tumour thickness <1.5mm) is 90%. However, for stage IIA (1.5 to 4.0mm), IIB (>4mm) and stage III (primary any size; nodal but no distant metastases) this drops to 70, 50 and 40%, respectively.[59] The reason is recurrent disease despite radical surgery.[58] Effective adjuvant treatment therefore would be expected to reduce relapses and improve survival.
rIFNα has been used widely in malignant melanoma since the 1980s. Following the publication of studies that showed response rates of about 15% in metastatic disease,[60–69] many randomised trials[70–77] have tried to establish the role of rIFNα in the adjuvant setting.[58] These are summarised in table III. They can be divided into three groups: those using high-dosage IFNα (HDIFN; ≥10MU), those using intermediate-dosage IFNα (IDIFN; 5 to 10MU) and those using low-dosage IFNα (LDIFN; ≤3MU).[78]
3.1.1 High-Dosage Trials
The first trial to show a benefit in OS for stage IIB/III disease, using HDIFN, was Eastern Cooperative Oncology Group (ECOG) EST 1684.[73] This recruited 287 patients with surgically resected high-risk melanoma. Patients were randomised to observation or HDIFN. This was given as induction (IFNα-2b 20 MU/m2/day intravenously for 5 days a week for 4 weeks) followed by maintenance (10 MU/m2/day subcutaneously thrice weekly for 48 weeks). After a 7-year follow-up, both disease-free survival (DFS) and OS were better in the IFNα arm (median OS 3.8 vs 2.8 years). The main drawbacks of HDIFN in this ‘Kirkwood regimen’ were the toxicity profile and the need for dosage reductions (~50% of patients), as two-thirdso experienced grade 3 to 4 toxicity. A retrospective analysis of quality of life concluded that the IFNα-treated group gained on average 8.9 months free of relapse (p = 0.03) and 7 months of OS (p = 0.02), against an average of 5.8 months of severe toxicity.[84] ECOG 1684 enabled the FDA to approve IFNα-2b as adjuvant therapy for stage IIB/III melanoma in 1995.[85]
The early data from ECOG EST 1684 and preliminary data from the World Health Organization (WHO)-16 trial,[70] ongoing at the time, suggested a role for HDIFN. Consequently, the intergroup trial ECOG 1690 followed.[76] This compared HDIFN with observation and with LDIFN as given in the WHO-16 study, but over 2 instead of 3 years. A preliminary analysis suggested no advantage in OS and borderline advantage in DFS (p = 0.05) for the HDIFN arm. For LDIFN there was no significant benefit in DFS or OS. This may have been due to improvements in survival of patients in the observation arm of ECOG 1690, who received IFNα salvage therapy.[58,78] Another study of HDIFN, North Central Cancer Treatment Group (NCCTG) 83–7052,[72] used IFNα at dosages as high as the Kirkwood regimen, but could not reproduce the results of ECOG 1684 or ECOG 1690. This may be related to the fact that IFNα was administered intramuscularly and only for 3 months.
Follow-up trials from ECOG 1684 and 1690 were E2696 and E1694. E2696 was a phase II trial that tested the effect of HDIFN and the GM2 ganglioside-based GMK vaccine on the induction of antibody responses. It enrolled 107 patients with resected stage IIB, III or IV melanoma into three arms: HDIFN + GMK given simultaneously (arm A) or sequentially (arm B) and GMK alone (arm C). The trial showed an improved DFS for the HDIFN + GMK vaccine arms (A and B). This finding must be viewed with caution because of the small sample size and the short follow-up time of 24 months.[79]
E1694 was a phase III intergroup study that compared HDIFN and the GMK vaccine. The National Cancer Institute’s (NCI) Drug Data Safety Monitoring Board halted this trial prematurely in May 2000 after significantly higher relapse and death rates in the vaccine arm. E1694 has now been published[80] and has reproduced the earlier findings of E1684 in demonstrating that HDIFN improves DFS and OS. Comparison of survival curves of the vaccine arm in E1694 showed this to be similar to the observation arm of E1690[86] and other adjuvant trials, suggesting that the vaccine was not worse than observation.[80]
An ongoing study, ECOG E1697, examines the role of adjuvant HDIFN for stage IIA-B patients (HDIFN, 20 MU/m2 five times a week for 4 weeks versus observation). Its aim is to establish the value of the initial induction phase with intravenous HDIFN,[87] when approximately 20% of the total yearly dose is delivered, as analysis of E1684 and 1690 appears to show that the effect of the high-dosage regimen appears to be early.[87] Another ongoing study is the Sunbelt melanoma trial, which is evaluating the role of the Kirkwood regimen in patients with histologically positive lymph nodes or reverse transcription-polymerase chain reaction (RT-PCR) positive nodes following sentinel node biopsy.[87,88] Finally, E1601 is aplanned study, which will compare 1 month of induction with HDIFN versus the full 1-year Kirkwood regimen in patients with microscopic lymph node involvement.[87]
3.1.2 Intermediate-Dosage Trials
EORTC 18952[58,85] tested IDIFN in AJCC stage IIB/III melanoma. This was administered during an induction phase (10 MU/day subcutaneously for 5 days per week over 4 weeks) followed by maintenance over 1 or 2 years (10 or 5MU three times weekly, respectively) and was compared with observation.[89] The first analysis of EORTC 18952 showed a benefit in distant metastasis-free interval (DMFI) for the 2-year IFNα arm (i.e. 5MU three times weekly). This benefit was statistically significant only for stage IIB disease.[89] Although IFNα at 5 to 10MU was well tolerated, the effect on OS was not significant. This may change with longer follow-up (so far, median 1.6 years).
The Scandinavian Melanoma Co-operative Group is conducting a similar trial with IDIFN as induction followed by maintenance with 10MU three times weekly for 1 or 2 years versus observation.[87] The German Dermatology Cooperative Oncology Group (DeCOG) initiated a trial of IDIFN versus LDIFN for stage II disease in 1997.[59] The absence of a direct comparison of IDIFN with HDIFN in this study may create problems in interpreting OS results if one of these IDIFN arms shows a survival advantage.[87]
3.1.3 Low-Dosage Trials
The first of these studies was WHO-16, which examined the role of LDIFN (3MU three times a week subcutaneously for 3 years) versus observation.[70,71] Neither the initial analysis of WHO-16 in 1994[70] nor a more recent analysis[90] showed any differences in DFS or OS between the LDIFN and observation groups. WHO-16 focused on recurrent nodal disease. It is possible that recurrent nodal disease is inherently resistant to IFNα, thus explaining no survival benefit when adjuvant IFNα is used in this setting. Alternatively, it is also possible that the lack of activity of IFNα may be related to the lower dosage used (approximately 40% of the total cumulative dose) compared with the Kirkwood regimen. The latter possibility, however, is difficult to reconcile in the light of two recent French and Austrian studies, which showed better DFS for LDIFN-treated patients (68 vs 58% and 72 vs 64%, respectively, at 3 years). In these two studies, IFNα was given for 12 to 18 months to patients with stage IIA-B melanoma, compared with 36 months in the WHO-16 study, where the patients had stage III disease (i.e. nodal metastases). These findings are difficult to explain. One could speculate that LDIFN (about 3MU three times weekly) given for long enough (>1 year) may be effective for just stage II disease. However, in the French study, the significant improvement in OS at 5 years has been lost with longer follow-up.[91] In contrast, IFNα may need to be given in much higher dosages during induction and maintenance therapy for patients with nodal metastases (stage III).
The UK Adjuvant Interferon in Melanoma High Risk (AIM High) study compared LDIFN given over 2 years and observation for stage IIB and III melanoma. Data presented at 2001’ s American Society of Clinical Oncology (ASCO) meeting failed to demonstrate a survival advantage in favour of LDIFN.[81] A smaller study from Scotland, which used IFNα 3MU three times weekly for just 6 months, also failed to show a survival benefit in stage II disease.[83] The lowest dosage of IFNα has been tested in the EORTC 18871 trial. This was a four-arm study in AJCC stage IIB/III disease. It compared LDIFN with IFNα, a control group and the homeopathic extract ‘Iscador’ given only within the DKG-80-1 German Cancer Society trial. LDIFN had no effect on DFS or OS.[89]
3.2 Metastatic Disease
3.2.1 Interferon-α Alone
Metastatic malignant melanoma has a poor survival of just 6 to 9 months.[92] Although dacarbazine is the most commonly used cytotoxic agent in metastatic malignant melanoma, responses are seen in just 15 to 25% of cases[69] and only a minority of these are complete or durable.[93] Investigators have therefore conducted numerous trials over the last 15 years in an attempt to improve response rates and survival. In the 1980s and early 1990s, many phase II studies (summarised in table IV) were conducted using IFNα in metastatic malignant melanoma.[60–68,94–99]
These trials yielded response rates of about 15% (range 0 to 38%) with about 5% complete response rate.[101,102] Median duration of response was short (6 to 9 months) and at best 12 months.[103,104] The interferon dosages used were 3 to 50 MU/m2/day three times weekly, with 10 to 20 MU/m2 being the most common dosage. Uninterrupted (i.e. daily or thrice weekly) administration of interferon was more effective than interrupted (i.e. cyclic) treatment,[102] and responses were seen with dosages between 10 and 50 MU/m2/day on alternate days.[101] Moreover, tumour response was primarily limited to patients with small volume or soft tissue disease (including skin).[105] This and localised lung disease were among the predictive factors for response.[106] The main toxicities were flu-like symptoms (fever, chills, fatigue, myalgia and headache), nausea, leucopenia and neuropsychiatric disturbances (mood swings, cognitive dysfunction).[106]
These trials were important because they demonstrated that, although the response rate to IFNα was comparable to that with chemotherapy alone, the responses in a small fraction of patients were durable.[58,69] Although in recent studies of chemotherapy for advanced malignant melanoma survival beyond 3 years is very rare,[107] several phase II studies have reported long-term responses of 3 to 5 years using rIFNα alone.[65,94–96] To our knowledge, there are no data from randomised studies comparing rIFNα alone with chemotherapy in metastatic malignant melanoma.
3.2.2 Interferon-α with Interleukin-2 or Interferon-γ
IL-2 was combined with IFNα because IL-2 alone has shown response rates of approximately 23% in phase II trials[108] and synergy with IFNα has been demonstrated in vitro.[106] In phase II studies of IFNα + IL-2, response rates ranged from 0 to 44% (table V).[106] Initially, LDIFN + low-dosage IL-2 given subcutaneously showed little activity. In a dose-escalating study, Rosenberg and colleagues[109] reported response rates as high as 44%. Kruit and co-workers[110] later reproduced these findings. The optimal regimen was found to be IFNα 3 MU/m2 + IL-2 11.7 MU/m2 intravenously every 8 hours for 5 days. These and other phase II studies suggested the existence of a dose-response relationship with IFNα + IL-2.[106] Unfortunately, the severe toxicities associated with the higher dosages precluded routine clinical use. This led to the development of the ‘decrescendo’ schedule by Keilholz et al.[111] This used a rapidly tapering dosage of IL-2 with IFNα 10 MU/m2/day subcutaneously on days 1 to 5, 4-weekly) and achieved a response rate of 41%. Unfortunately, the only randomised trial, to our knowledge, of IFNα + IL-2 versus IL-2 alone was disappointing, with low response rates of 10 and 5%, respectively, and no survival difference.[112] IFNα has also been used with IFNγ and found to be ineffective.[113]
3.2.3 Interferon-α with Single-Agent Chemotherapy
IFNα has been combined with cytotoxic chemotherapy, mainly dacarbazine (table VI) and cisplatin. Dacarbazine is the most tested cytotoxic agent in melanoma with one of the highest response rates (approximately 20%).[118] One small randomised trial of 61 patients showed a significant improvement in response rate and survival for the IFNα + dacarbazine combination.[119] Three more studies involving more than 650 patients failed to confirm the earlier findings.[120–122] Consequently, the combination of IFNα with dacarbazine cannot be recommended.[106]
Cisplatin is one of the other active agents in metastatic malignant melanoma, with response rates of 15 to 25%.[118] A small phase II study showed that IFNα + cisplatin was tolerated well and that some synergism may exist between them.[123] Despite encouraging preclinical data, phase I/II studies involving about 100 patients have reported response rates of 7 to 14%,[118] with no improvement in survival.[123,124]
3.2.4 Interferon-α with Combination Chemotherapy
Several phase II trials in which 317 patients have been treated have demonstrated improved response rates (26 to 68%, mean 44%) when IFNα is combined with several cytotoxic agents (table VII).[125–131] Pyrhonen and colleagues[125] reported one of the highest response rates; however, nearly half of their patients had stage III disease (i.e. nodal metastases), which carries a better prognosis than stage IV disease.[106] The combination of IFNα with dacarbazine and fluorouracil protected against central nervous system relapse in responding patients,[132] and this should be evaluated further. Nevertheless, none of these studies showed improvement in survival, and because of the enhanced toxicity seen with some regimens the use of IFNα with combination chemotherapy outside a clinical trial is not recommended.
Phase II trials of interferon-α with combination chemotherapy in metastatic melanoma (reproduced from Legha,[69] with permission)
3.2.5 Interferon-α with Chemoimmunotherapy
IFNα has been combined with conventional cytotoxic agents with or without IL-2 and tested in more than 900 patients in phase III studies (table VIII).[125,132–137] Response rates have varied from 16 to 48% and median survival has been approximately 1 year. Over the last 10 years, some randomised studies[138–142] have compared IFNα-based immunotherapy with immunotherapy + chemotherapy. In three of six randomised trials of chemoimmunotherapy involving nearly 400 patients, there was a trend for a higher response rate (table VIII) in the IFNα or IFNα + IL-2 arms. However, none of these or any of the other studies demonstrated a survival advantage for either arm. In the 1997 study by Keilholz and co-workers,[139] addition of cisplatin to IFNα and IL-2 enhanced the response rate but did not affect survival, whereas in the study by Rosenberg and colleagues[141] (cisplatin, dacarbazine, tamoxifen ± IFNα and IL-2) there was no statistical difference in response rate or survival. More recently, the Dermatologic Cooperative Oncology Group reported on a randomised trail of IFNα with dacarbazine ± IL-2 and showed no difference in response rate or OS.[107]
The sequence of administration may be important. Platinum-containing chemotherapy given preferably before or concurrently with immunotherapy has produced the best results. In a phase II study of 60 patients (30 per group) where chemotherapy was followed by immunotherapy, the response rate was 73%; if immunotherapy preceded chemotherapy the response rate was 47%.[135] This is not surprising, as conventional cytotoxic agents reduce disease volume, thus facilitating the subsequent antitumour function of immune effector cells. For concurrent chemoimmunotherapy the response rate in 52 patients was 63%.[135] More recent data have shown the response rate of concurrent chemoimmunotherapy and sequential chemotherapy-immunotherapy to be 62 and 60%, respectively.[137]
rIFNα has been tested with a variety of other agents such as cimetidine,[143] eflornithine (difluoromethylornithine),[144] low-dose cyclophosphamide,[145] retinoids[146,147] and indomethacin.[148] None of these has shown a potential benefit. Temozolomide has been tried in a phase I study with some responses.[149]
4. Renal Cell Carcinoma
IFNα has been extensively investigated in both the metastatic and adjuvant setting of renal cell carcinoma (RCC). RCC has a unique and complex natural history and can remain unrecognised during the lifetime of the affected individual, even after metastatic spread.[150,151] Even when clinically manifest with metastasis, RCC can undergo rare but well documented spontaneous remissions.[152,153] These facts indicate the ability of the host immune system to control RCC under certain, unique but undefined, cellular conditions. This, along with the evidence of efficacy of active specific immunotherapy, gave impetus to the use of IFNα in the management of RCC.[154]
4.1 Metastatic Disease: Monotherapy
IFNα was found to be active against RCC as early as 1983.[155] In vitro studies have also shown that the interferons have a direct antiproliferative effect on renal tumour cells.[156,157] The response rates for metastatic RCC, in various phase II studies, range from 0 to 31% (table IX). Even though most of the responses are partial responses and only a few are durable complete responses, the responding patients were found to have significant prolongation of progression-free survival. These results led to the evaluation of IFNα in randomised phase III trials, which have confirmed the efficacy of IFNα in metastatic RCC. However, the response rates in the randomised studies are only in the region of 10 to 15%.[158] One of the early randomised trials using an IFNα dose of 2MU found no benefit for IFNα compared with medroxyprogesterone.[159] The negative result was probably due to the low dose of IFNα used in the trial, since a previous phase II study and a randomised trial had shown lack of benefit for LDIFN compared with HDIFN.[160,161] Another randomised trial in the 1990s also failed to confirm the beneficial effects of IFNα.[162]
The first major randomised trial to demonstrate a survival benefit for IFNα monotherapy in metastatic RCC was the Medical Research Council (MRC) RE 01 trial. This trial randomised patients with advanced RCC to either medroxyprogesterone or IFNα.[170] A total of 350 patients were randomly assigned to receive treatment, and by the time early results were published 236 patients had died of disease, with 90% dying of metastatic RCC. There was an OS advantage for IFNα, with a hazard ratio of 0.72 (0.55 to 0.94). The absolute improvement in 1-year survival for the interferon group was 12% (43 vs 31%). The improvement in median survival of 2.5 months due to IFNα has to be viewed against significant toxicity, namely tiredness, nausea and lack of energy, which may impair quality of life. The recently updated results have confirmed the results of earlier analysis.[171]
The modest efficacy of IFNα in metastatic RCC has to be balanced against treatment-related toxicity. An attempt is being made to improve the therapeutic ratio of interferon by modifying its structure. One such modified molecule is pegylated interferon (peginterferon-α), which after subcutaneous administration results in sustained absorption. Peginterferon-α is IFNα modified by the covalent attachment of polyethylene glycol polymer molecules; this renders it less immunogenic and prolongs its half-life.[172] A recent phase II trial of peginterferon-α in 40 patients has shown an response rate of 13% and favourable toxicity profile, and hence phase III trials are planned.[173]
4.2 Metastatic Disease: Combination Therapy
Even though IFNα produces a small but significant survival advantage in the treatment of metastatic RCC, the response rates have been fairly modest (<20%), particularly in the randomised trials. This has led to attempts to integrate IFNα with other biological agents and conventional chemotherapy agents. The widely evaluated combinations are IFNα + IL-2, and the addition of cytotoxic agents to IFNα (table X).
4.2.1 Interferon-α with Interleukin-2
IL-2 is predominantly used for metastatic RCC in North America, whereas IFNα is used preferentially in Europe. Both have only modest efficacy, and hence attempts are being made to give them in combination, with a view to improving the outcome of RCC. The combination of high-dosage IL-2 intravenously and IFNα subcutaneously produces significant toxicity.[168,209] Hence, alternative strategies were explored to reduce toxicity. One such approach was subcutaneous administration of low-dosage IL-2, which is associated with less toxicity.[175,176,210] Even so, the combination of subcutaneous IL-2 and IFNα has also been found to have significant toxicity in a few studies. Moreover, the low dosages of the cytokines could compromise efficacy of the regimen.[182,211,212]
One early, multicentre, phase II study assessed the feasibility of combining IL-2 with IFNα using an outpatient low-dosage subcutaneous regimen. This study involved 42 patients with immunotherapy-naive metastatic RCC and found an response rate of 12% with mild toxicity and a median survival of 14.5 months.[176] The response rates of up to 30% in various pilot studies led to prospective randomised studies of the IFNα + IL-2 combination.[174] The results from the prospective randomised studies did not confirm the results of the pilot studies.[178–182]
A multicentre randomised French trial[183] provides interesting data regarding the IFNα + IL-2 combination. In this trial, 420 patients were randomly assigned to continuous intravenous IL-2 or IFNα, or both. After a median follow-up of 39 months, the OS was not significantly different between the three groups even though the response rate in the combined arm was more than twice the response rate in the single-drug arms. The response rates in the single arms involving IL-2 and IFNα were low, at about 7 and 8%, respectively. Moreover, toxicity was higher in the IL-2 arms. This trial effectively rules out the use of this combination in the routine clinical setting as well as in the trial setting. Moreover, the toxicity profile in the three arms of this trial favours the use of IFNα over IL-2 in the routine clinical setting.[183]
The combination of low-dosage IL-2 and IFNα has been compared with high-dosage IL-2 in a recent phase III randomised trial.[184] A total of 193 patients were randomised in this trial and the preliminary results, in an abstract, show favourable response rates in the high-dosage IL-2 arm (25 vs 12%), thereby suggesting that the combination of subcutaneous low-dosage IL-2 and IFNα is unlikely to produce superior survival benefits.[184]
Attempts are also being made to improve the therapeutic ratio of the IFNα + IL-2 combination by adding a chemotherapeutic agent. The drawbacks of this approach are that the increased toxicity compromises the cytokine dosage and the regimen affects the quality of life of patients who essentially have incurable disease. A phase II study assessing the benefit of adding vinblastine to the combination of IFNα + IL-2 showed a response rate of 38%. About 50% of patients experienced grade 2 to 3 neutropenia, but this did not warrant dosage modification. These results need further investigation in a randomised trial.[198]
A few trials have examined the value of adding isotretinoin (13-cis-retinoic acid) to the combination of IFNα and IL-2, but the value of this approach remains uncertain.[206,213]
4.2.2 Interferon-α with Interleukin-2 and Fluorouracil
The combination of fluorouracil, IFNα and IL-2 was pioneered by Atzpodien’s group.[185] This outpatient combination therapy in a small trial of 35 patients produced an response rate of 48% (complete response 11% + partial response 37%), with a further 37% having stable disease. Moreover, the systemic toxicity in this trial was only mild to moderate. Although many other groups have noted similar results for this combination,[186,188,189,191,193] recent studies, including a randomised trial, have not confirmed its superior response rate and better tolerability.[177,187,190,192,194–197,214,215] The randomised phase III MRC RE-04 trial, which is comparing the three-drug ‘Atzpodien’ regimen with single-agent IFNα, will clarify whether this combination is superior to monotherapy in metastatic RCC. The combination of fluorouracil + IFNα + IL-2 is also being tested in an EORTC adjuvant trial of high-risk RCC patients.
4.2.3 Interferon-α with Vinblastine
The preclinical data suggesting synergism between IFNα and vinblastine led to pilot clinical studies that showed response rates of 16 to 44%.[216–218] Conversely, an early prospective study and two randomised studies failed to demonstrate any significant benefit for the combination.[199,219,220] By contrast, the recently published trial by Pyrhonen and colleagues[200] was the first trial to demonstrate survival benefit for combination therapy in metastatic RCC. This prospective randomised trial demonstrated that the combination of IFNα, at a dosage of 18MU three times weekly, with vinblastine was superior to the control arm of vinblastine alone. This trial randomised 160 patients and the median survival of patients receiving combination therapy was 67 weeks as opposed to 37 weeks in the control arm. Unlike this trial, an earlier European multicentre study, using IFNα as the control, found no survival benefit for the combination of IFNα and vinblastine, even though there was a doubling of the response rate in the combination arm.[199]
4.2.4 Interferon-α with Retinoids
Preclinical studies demonstrating synergism between IFNα and the retinoids[221] led to phase II trials in which response rates of up to 30% were noted for this combination.[222] However, a recent randomised study has not shown any significant benefits in terms of response rate or OS for the addition of isotretinoin to IFNα.[223] A positive aspect of this trial was that the duration of response and progression-free survival were longer in the combination arm, but this came at the price of poor quality of life.[223] A more recent study combined LDIFN with isotretinoin and weekly paclitaxel chemotherapy in a group of patients with advanced RCC, some of whom were pretreated with IL-2 and/or IFNα. Although this treatment was well tolerated, its efficacy was minimal.[224]
4.2.5 Interferon-α with Interferon-γ
Once again, preclinical studies suggesting synergism between IFNα and IFNγ led to clinical trials evaluating this combination.[225] An EORTC phase III trial exploring this combination failed to show beneficial effects.[202] An earlier small randomised study by Foon and coworkers[226] again showed a lack of benefit for the combination. Together, these studies confirm the lack of synergism between IFNα and IFNγ in metastatic RCC.
4.2.6 Interferon-α with Fluorouracil
The combination of IFNα and fluorouracil showed response rates of 43% in one of the early studies,[205] but many other studies suggest that this combination is unlikely to produce a better outcome in patients with metastatic RCC.[227,228]
4.2.7 Other Combinations
IFNα has been combined with many other agents in various phase I/II trials with mixed and inconclusive results. Randomised studies exploring the addition of cimetidine + coumarin, or aspirin (acetylsalicylic acid), to IFNα found no difference in response rate or OS.[203,204] Histamine protects immune cells (NK and T cells) by inhibiting the formation and release of reactive oxygen species, and hence it is being assessed in combination with IFNα and IL-2. The phase II results are interesting and further trials are needed before firm conclusions can be drawn.[208]
4.3 Dose Response, Treatment Duration and Late Responses
The dose of IFNα used in the various studies of RCC ranged from 1 to 30MU, typically given three times a week by subcutaneous route. The lower doses have been found to be ineffective in phase II and phase III trials.[160,161,167] The efficacy of IFNα reaches a plateau after doses of about 10MU, and higher doses are associated with inferior response rates because of poor tolerability.[168] A bell-shaped curve has been suggested for the dose-response curve.[229] Hence, IFNα doses of the order of 5 to 10MU are commonly used in the clinical setting.
The optimal duration of IFNα treatment is yet to be well defined. The MRC RE 01 randomised trial, which was the first to demonstrate a survival benefit for IFNα therapy, used an IFNα schedule of 10MU three times weekly for 12 weeks only. But the evidence indicates that prolonged IFNα therapy would be beneficial. Recently, in a phase II trial of 75 patients, Kankuri and colleagues[230] observed an response rate of 17% (43% stable disease) with the use of an intermittent schedule of IFNα therapy [treatment with maximum tolerated dose (MTD) with escalating doses of 4.5 to 18MU, three times weekly, with 1 week rest each month]. Significantly, they observed three late objective responses (4%) after a long period of stable disease (12 to 16 months). The responding patients were noted to have prolonged median time to response (median 7 months; range 4 to 16 months). Despite the monthly interruption of treatment for a week, there was no apparent adverse effect on the response rates and survival rates. The median duration of response was 16 months, and indirect comparisons indicate that the survival rates are better than those previously reported with continuous IFNα regimens.[231,232] Moreover, the objective responses were noted only in patients treated with 9MU, and 88% of the patients tolerated an IFNα dosage of at least 9MU.[230] This trial demonstrates the need for prolonged IFNα therapy (>12 months) at MTD, not only in patients with objective response but also in those with stable disease.
Buzio and coworkers[233] provide further support for prolonged IFNα therapy in RCC. In a phase II trial of 50 patients, they administered low-dosage IL-2 (1 MU/m2, days 1 to 5) and IFNα (1.8 MU/m2, days 3 and 5) for 4 consecutive weeks every 4 months, irrespective of response. The overall response rate was 12%. They observed four late responses (8%) after a period of 14 to 16 months in patients who were treated with immunotherapy despite progressive disease. Hence, IFNα therapy should be administered indefinitely to patients with responding or stable disease. The role of low-dosage intermittent immunotherapy needs further evaluation in large randomised studies.
4.4 Adjuvant Therapy
About 15 to 50% of patients with localised RCC will eventually relapse. In particular, a majority of patients with resectable but locally advanced node-positive tumours will relapse eventually.[234–237] Since IFNα has modest efficacy in metastatic RCC, studies were initiated to assess the effect of IFNα in the adjuvant setting, where it was hoped to be more effective because of the low tumour burden. The early pilot studies showed a significant increase in host NK cell activity after initiation of IFNα therapy. This immune modulation was expected to reduce the risk of recurrence in high-risk patients after nephrectomy.[238] However, randomised studies of IFNα in the adjuvant setting have not shown any survival benefits or reduction in recurrence rates.[239–242]
5. Summary and Conclusions
5.1 Malignant Melanoma
A recent meta-analysis of the four ECOG trials of HDIFN[243] has reported a beneficial effect of HDIFN on DFS and OS. One must, however, take into account the fact that the median follow-up time for E2696 and E1694, which together account for nearly half of all patients, was relatively short (22 and 29 months, respectively). More importantly, at median follow-up of 12 years, the OS benefit for the HDIFN arm in E1684 has been lost.[78] When only data from mature HDIFN trials are considered, it appears that HDIFN prolongs DFS[78] (supported by the E1684,E1690 and E1694 data) but not OS (supported by E1690 and the latest data from E1684). The recent results of E1694 could provide support for HDIFN; however, in addition to the above, two important issues remain to be resolved. First, the E1694 data must be allowed to mature and, secondly, reproducibility of the E1694 findings is essential before a change in clinical practice occurs. Moreover, the toxicity and financial costs associated with HDIFN are so significant that there must be unequivocal evidence for the benefit of such treatment before it is universally applied.
A meta-analysis done by the UKCCCR includes data from trials comparing adjuvant IFN with observation (about 3000 patients in total). The meta-analysis, which included data from EORTC 18871, the AIM High study and the Scottish study, showed a statistically significant benefit in DFS (p = 0.00001) and OS (p = 0.02).[58] A more recent meta-analysis[82] that included data from ten randomised trials (about 3700 patients), and included the latest AIM High results,[81] has again shown a benefit in DFS in favour of IFNα [odds ratio (OR) 0.84, 95% confidence interval (CI) 0.77 to 0.92, p = 0.0001]. For OS, however, the benefit was less clear (OR 0.90, 95% CI 0.82 to 1.00, p = 0.05). Interestingly, there was no statistically significant evidence of benefit of HDIFN versus LDIFN. As far as IDIFN is concerned, no conclusions can be reached until the results of EORTC 18952 mature. The ongoing EORTC 18991 is a randomised study evaluating long-term administration of peginterferon-α (administered once weekly for 5 years) in stage III disease compared with observation.[91] LDIFN has not been shown in any study to prolong OS, and therefore its routine clinical use outside a clinical trial cannot be recommended. Others have echoed this view even before the publication of the AIM High study.[58,106,244] A more recent meta-analysis[245] of eight randomised, controlled clinical trials of adjuvant LDIFN or HDIFN in which 3178 patients were treated has shown no clear benefit in OS. Some investigators believe that adjuvant HDIFN should be considered standard therapy for high-risk patients who are likely to tolerate it.[246] However, the absence of a convincing benefit on OS as demonstrated by the two recent meta-analyses[82,245] would not be supportive of the use of adjuvant IFN outside a clinical trial.
In advanced melanoma, the in vitro data that showed synergism between IL-2 and IFNα[31,247] have not translated into a clear-cut survival benefit. On current evidence the IFNα + IL-2 combination cannot be recommended for routine clinical use. What has emerged, however, is that a small percentage of patients treated with IFNα + IL-2 will have durable responses.[69,108] In one study, nearly 40% of patients survived for more than 3 years.[248] One of the challenges for the next few years, therefore, will be to establish tumour and patient characteristics that predict long-term responders.
Chemoimmunotherapy (‘bio-chemotherapy’) has achieved high response rates[69,106,108] (40 to 55%), and high complete response and long-term non-maintained remission rates (5 to 15%).[118] An analysis of trials that included regimens with IFNα + IL-2 showed a 2.5-month survival advantage compared with chemotherapy alone.[249] However, a more recent retrospective analysis of 15 phase II/III trials suggested that IFNα had no effect on OS.[250] Chemoimmunotherapy regimens may increase toxicity significantly,[251] and as their benefits are unproven[8,106] routine use outside prospective randomised trials cannot be recommended.[251] The currently ongoing study ECOG 3695/CALGB 509802 by the Eastern Cooperative Oncology Group and the Cancer and Leukaemia Group B comparing cisplatin, vinblastine and dacarbazine (CVD) chemotherapy with CVD + IL-2 + IFNα may give some answers.[8] Although the role played by IFNα in the induction and maintenance of remission with bio-chemotherapy is still unknown, the above information should be taken into consideration in the design of future phase III randomised trials.
5.2 Renal Cell Carcinoma
IFNα has definite but modest activity in metastatic RCC. Significant long-term survival occurs in a small cohort of patients as a result of IFNα treatment.[252] This has to be balanced against the toxicity of the treatment, the impairment in quality of life of many to benefit a few and, importantly, the high cost of IFNα therapy. Hence, proper selection of patients for treatment is very important. The response to IFNα is associated with various prognostic factors such as good performance status, solitary or fewer metastatic sites, prior nephrectomy and low erythrocyte sedimentation rate.[169,253,254] These factors should guide the initiation of IFNα treatment in metastatic RCC. Of special importance is the issue of prior nephrectomy. Two randomised studies[255,256] demonstrated that nephrectomy prior to immunotherapy with IDIFN (5 MU/m2 subcutaneously three times weekly), in patients with advanced RCC confers a survival benefit and delays time to progression. Hence, nephrectomy should be considered at least in patients with good performance status (WHO 0 or 1).[257]
In the adjuvant setting, there is no evidence for use of IFNα outside clinical trial protocols. Similarly, the evidence is also weak for use of IFNα in combination with IL-2 and other chemotherapy agents. Peginterferon-α may have the potential to improve the therapeutic ratio of IFNα treatment, and this needs assessment in large randomised trials.[173]
The second part of this review[258] will deal with the use of IFNα in solid tumours where it has demonstrated activity, such as superficial bladder cancer and Kaposi’ s sarcoma. Part 2 will also cover head and neck cancer, gastrointestinal malignancies, common solid tumours such as lung and breast cancer, and gynaecological malignancies.
References
Isaacs A, Lindenmann J. Virus interference I: the interferon. Proc R Soc Lond Ser B 1957; 147:258–67
Nagano Y, Kojima Y. Inhibition de l’infection vaccinale par un facteur liquide dans le tissu infecte par le virus homologue. C R Soc Biol 1958; 152: 1627–9
Pestka S. The human interferon-alpha species and hybrid proteins. Semin Oncol 1997; 24 3 Suppl. 9: S9–4–S9–17
Walter MR, Bordens R, Nagabhushan TL, et al. Review of recent developments in the molecular characterization of recombinant alfa interferons on the 40th anniversary of the discovery of interferon. Cancer Biother Radiopharm 1998; 13(3): 143–54
Zav’yalov V, Zav’yalova GA. Interferons α/β and their receptors: place in the hierarchy of cytokines. APMIS 1997; 105: 161–86
Ruszczak Z, Schwartz RA. Interferons in dermatology: biology, pharmacology, and clinical applications. Adv Dermatol 1997; 13: 235–88
Hanley JP, Haydon GH. The biology of interferon-alpha and the clinical significance of anti-interferon antibodies. Leuk Lymphoma 1998; 29(3-4): 257–68
Jonasch E, Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications and toxicities. Oncologist 2001; 6(1): 34–55
Tossing G. New developments in interferon therapy. Eur J Med Res 2001; 6(2): 47–65
Cantell H, Hirovonen S, Mogensen KE, et al. Human leucocyte interactions: production, purification and stability experiments. The production and use of interferon for the treatment of human virus infections. In vitro monograph 3. Rockville (MD): Tissue Culture Association, 1984: 35–8
Rubinstein M, Rubinstein S, Familletti PC, et al. Human leucocyte interferon: production, purification to homogeneity and initial characterization. Proc Natl Acad Sci U S A 1979; 76: 640–4
Stewart WE, Blalock JE, Burke DC. Interferon nomenclature. Nature 1980; 286: 110
Pestka S. The human interferons: from protein purification and sequence to cloning and expression in bacteria: before, between and beyond. Arch Biochem Biophys 1983; 221(1): 307–18
Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon producing cells in human blood. Science 1999; 284(5421): 1835–7
Goeddel DV, Leung DW, Dull TJ, et al. The structure of eight distinct human cloned human leucocyte interferon cDNAs. Nature 1981; 290(5801): 20–6
Streuli M, Nagata S, Weissman C. At least three human type a interferons: structure of a 2. Science 1980; 209: 1343–7
Maeda S, McCandliss R, Gross M, et al. Construction and identification of bacterial plasmids containing nucleotide sequence for human leukocyte interferon. Proc Natl Acad Sci U S A 1980; 77(12): 7010–3
Nagata S, Taira H, Hall A, et al. Synthesis in E. coli of a polypeptide with human leukocyte interferon activity. Nature 1980; 284(5754): 316–20
Pfeffer LM. Biologic activities of natural and synthetic type I interferons. Semin Oncol 1997; 24 3 Suppl. 9: S–9–63–9
Pestka S. The interferon receptors. Semin Oncol 1997; 24 3 Suppl. 9: S9–18–40
Williams BRG, Haque SJ. Interacting pathways of interferon signalling. Semin Oncol 1997; 24 3 Suppl. 9: S9–70–7
Pestka S, Trotta PP. Recombinant alfa-interferons from naturally occurring genes: introduction. Semin Oncol 1997; 243 Suppl. 9: S9–1–3
Herberman RB. Effects of α-interferons on immune function. Semin Oncol 1997; 243 Suppl. 9: S9–78–80
Der SD, Zhou A, Williams BR, et al. Identification of genes differentially regulated by interferon alpha, beta, or gamma using oligonucleotide arrays. Proc Natl Acad Sci U S A 1998; 95: 15623–8
Pitha PM. Introduction: interferons’ connection to cancer. Semin Cancer Biol 2000; 10(2): 69–72
Barber GN. The interferons and cell death: guardians of the cell or accomplices of apoptosis? Semin Cancer Biol 2000; 10(2): 103–11
Wells V, Mallucci L. Expression of the 2-5A system during the cell cycle. Exp Cell Res 1985; 159:27–36
Sekar V, Atmar VJ, Joshi AR, et al. Inhibition of ornithine decarboxylase in human fibroblast cells by type I and type II interferons. Biochem Biophys Res Commun 1983; 114: 950–4
Contente S, Kenyon K, Rimoldi D, et al. Expression of gene rrg is associated with reversion of NIH3T3 transformed by LTR-c-H-ras. Science 1990; 249: 796–8
Fleischmann WRJ, Newton RC, Fleischmann CM, et al. Discrimination between non-malignant and malignant cells by combinations by IFN γ and IFN α/β. J Biol Response Mod 1984; 3: 397–405
Giacomini P, Aguzzi A, Pestka S, et al. Modulation by recombinant DNA leukocyte (alpha) and fibroblast (beta) interferons of the expression and shedding of HLA- and tumour-associated antigens by human melanoma cells. J Immunol 1984; 133(3): 1649–55
Baron S, Tyring SK, Fleischmann RJ, et al. The interferons: mechanisms of action and clinical applications. JAMA 1991; 266(10): 1375–83
Kurzrock R, Gutterman JU, Talpaz M. Interferons-α, β, γ: basic principles and preclinical studies. In: De Vita VT, Hellman S, Rosenberg SA, editors. Biologic therapy of cancer. Philadelphia (PA): JB Lippincott, 1991: 247–74
Chen L, Tourvielle B, Burns GF. Interferon: a cytotoxic T-lymphocyte differentiation signal. Eur J Immunol 1986; 16: 767–70
Folkman J. Antiangiogenic therapy. In: de Vita Jr VT, Hellman S, Rosenberg SA, editors. Principles and practice of oncology. 5th ed. Philadelphia (PA): Lippincott-Raven, 1997: 3075–85
Sidky YA, Borden EC. Inhibition of angiogenesis by interferons: effects on tumor-and lymphocyte-induced vascular responses. Cancer Res 1987; 47: 5155–61
White CW, Sondheimer HM, Crouch EC, et al. Treatment of pulmonary hemangiomatosis with recombinant interferon alfa-2a. N Engl J Med 1989; 320: 1197–200
Orchard PJ, Smith CM, Woods WG, et al. Treatment of haemangioendotheliomas with alpha interferon. Lancet 1989; II: 565–7
Folkman J. Successful treatment of an angiogenic disease. N Engl J Med 1989; 320: 1211–2
Ezekowitz RAB, Mulliken JB, Folkman J. Interferon alfa-2a therapy for lifethreatening hemangiomas of infancy. N Engl J Med 1992; 326(22): 1455–63
Mosmann TR, Cherwinski H, Bond MW, et al. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986; 136(7): 2348–57
Tompkins WA. Immunomodulation and therapeutic effects of oral use of interferon-alpha: mechanism of action. J Interferon Cytokine Res 1999; 19(8): 817–28
Herberman RB, Ortaldo JR, Bonnard GD. Augmentation by interferon of human natural and antibody-dependent cell-mediated cytotoxicity. Nature 1979; 277: 221–3
Strander H, Cantell K, Jakobson PA, et al. Exogenous interferon therapy of osteogenic sarcoma. Acta Orthop Scand 1974; 45: 958–67
Strander H. Interferons: antineoplastic drugs? Blut 1977; 35: 279–99
Culliton BJ, Waterfall WK. War on cancer -interferon. BMJ 1979; 2: 195–6
Ikic D, Krusic J, Cupak K, et al. The use of human leukocytic interferon in patients with cervical cancer and baseocellular cancer of the skin. Proceedings of the Symposium on Clinical Use of Interferon, Yugoslav Academy of Sciences and Arts; 1975 Oct 1-2; Zagreb, 239-44
Merigan TC, Sikora K, Breeden JH, et al. Preliminary observations on the effect of human leukocyte interferon in human non-Hodgkin’s lymphoma. N Engl J Med 1978; 299: 1449–53
Gutterman J. Phase I-II study of leucocyte interferon for recurrent breast cancer. NIAID/NCI workshop on clinical trials with exogenous interferon, National Institutes of Health; 1978 Mar 21-23; Bethesda
Gutterman JU, Blumenschein GR, Alexanian R, et al. Leucocyte interferon-in-duced tumour regression in human metastatic breast cancer, multiple myeloma, and malignant lymphoma. Ann Intern Med 1980; 93(3): 399–406
Maricic Z, Nola P, Ikic D, et al. Human leukocyte interferon in therapy of patients with urinary bladder papillomatosis, breast cancer, and melanoma. J Cancer Res Clin Oncol 1981; 10(3): 317–23
Ddc D, Krusic J, Kirhmajer V, et al. Application of human leucocyte interferon in patients with carcinoma of the uterine cervix. Lancet 1981; I(8228): 1027–30
Borden EC, Holland JF, Dao TL, et al. Leucocyte-derived interferon (alpha) in human breast carcinoma. Ann Intern Med 1982; 97(1): 1–6
Spiegel RJ. Clinical overview of alpha interferon: studies and future directions. Cancer 1987; 59: 626–31
Kurzrock R, Talpaz M, Gutterman JU. Interferons: clinical applications. In: DeVita VT, Hellman S, Rosenberg SA, editors. Biologic therapy of cancer. Philadelphia: JB Lippincott, 1991: 334–45
John WJ, Foon KA. Interferon use in solid tumors. Cancer Treat Res 1998; 94:23–33
Borden EC, Lindner D, Dreicer R, et al. Second-generation interferons for cancer: clinical targets. Semin Cancer Biol 2000; 10: 125–44
Hancock BW, Harris S, Wheatley K, et al. Adjuvant interferon-alpha in malignant melanoma: current status. Cancer Treat Rev 2000; 26(2): 81–9
ERROR: Invalid Float tag Cascinelli N. Therapeutic advances in melanoma: cutaneous melanoma, current status: Melanoma Congress Report Series. 3rd International Conference on the Adjuvant Therapy of Malignant Melanoma; 1999 Mar 19-20; London, 2-6
Creagan ET, Ahman DL, Green SJ, et al. Phase II study of recombinant leukocyte A interferon (r-IFN A) in disseminated malignant melanoma. Cancer 1984; 54: 2844–9
Creagan ET, Ahman DL, Green SJ, et al. Phase II study of low-dose recombinant leukocyte A interferon in disseminated malignant melanoma. J Clin Oncol 1984; 2: 1002–5
Hersey P, Hasic E, MacDonald M, et al. Effects of recombinant leucocyte interferon (rIFN-alpha A) on tumour growth and immune responses in patients with metastatic melanoma. Br J Cancer 1985; 51(6): 815–26
Coates A, Railings M, Hersey P, et al. Phase II study of recombinant alpha 2-interferon in advanced malignant melanoma. J Interferon Res 1986; 6: 1–4
Dorval T, Palangie T, Jouve M, et al. Clinical phase II trial of recombinant DNA interferon (interferon alpha 2b) in patients with metastatic malignant melanoma. Cancer 1986; 58(2): 215–8
Legha SS, Papadopoulos NE, Plager C, et al. Clinical evaluation of recombinant interferon alpha-2a (Roferon A) in metastatic melanoma using two different schedules. J Clin Oncol 1987; 5(11): 1240–6
Mughal JE, Robinson WA, Thomas MR, et al. Role of recombinant interferon-alpha-2 in treatment of advanced malignant melanoma [abstract]. Proc Am Soc Clin Oncol 1988; 7: 25
Neefe JR, Legha SS, Markowitz A, et al. Phase II study of recombinant alpha-interferon in malignant melanoma. Am J Clin Oncol 1990; 13(6): 472–6
Mickiewicz E, Estevez R, Rao F, et al. Interferon-alpha-2b (rIFN-alpha-2b) alone or in combination with DTIC in metastatic melanoma (MM): compiled data [abstract]. Proc Am Soc Clin Oncol 1990; 9: 281
Legha SS. The role of interferon alfa in the treatment of metastatic melanoma. Semin Oncol 1997; 24 1 Suppl. 4: 24–31
Cascinelli N, Bufalino R, Morabito A, et al. Results of adjuvant interferon study in WHO melanoma programme. Lancet 1994; 343: 1287–93
Cascinelli N. Evaluation of efficacy of adjuvant rIFNa2a in melanoma patients with regional node metastases [abstract]. Proc Am Soc Clin Oncol 1995; 14: 410
Creagan ET, Dalton RJ, Ahmann DL, et al. Randomized surgical adjuvant trial of recombinant interferon-alfa-2a in selected patients with malignant melanoma. J Clin Oncol 1995; 13(11): 2776–83
Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferonα-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Co-operative Oncology Group trial EST 1684. J Clin Oncol 1996; 14(1): 7–17
Grob JJ, Dreno B, de la Salmoniere P, et al. Randomised trial of interferonα-2a as adjuvant therapy in resected primary melanoma thicker than 1-5mm without clinically detectable nodal metastases. Lancet 1998; 351(9120): 1905–10
Pehamberger H, Sayer HP, Steiner A, et al. Adjuvant interferon alpha-2a treatment in resected primary stage II cutaneous melanoma. J Clin Oncol 1998; 16(4): 1425–9
Kirkwood JM, Ibrahim J, Sondak V, et al. Preliminary analysis of the E1690/ S9111/C9190 intergroup postoperative adjuvant trial of high- and low-dose IFNα2b (HDI and LDI) in high-risk primary or lymph node metastatic melanoma [abstract]. Proc Am Soc Clin Oncol 1999; 18: 537a
Kleeberg UR, Brocker FB, Lejeune F, et al. Adjuvant trial in melanoma patients comparing rIFN-α to rIFN-β to Iscador to a control group after curative resection of high risk primary (>3mm) or regional lymph node metastasis (EORTC 18871) [abstract 264]. Eur J Cancer 1999; 35 Suppl. 4: S82
Punt CJA, Eggermont AMM. Adjuvant interferon-alpha for melanoma revisited. Ann Oncol 2001; 12: 1663–6
Kirkwood JM, Ibrahim J, Lawson DH, et al. High-dose interferon alfa-2b does not diminish antibody response to GM2 vaccination in patients with resected melanoma: results of the multicenter Eastern Cooperative Oncology Group phase II trial E2696. J Clin Oncol 2001; 19(5): 1430–6
Kirkwood JM, Ibrahim JG, Sosman JA, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: results of intergroup trialE1694/S9512/C509801. J Clin Oncol 2001; 19(9): 2370–80
Hancock BW, Wheatley K, Harrison G, et al. Aim High-adjuvant interferon in melanoma (high risk), A United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) randomised study of observation versus adjuvant low dose extended duration interferon alpha-2a in high risk resected malignant melanoma [abstract]. Proc Am Soc Clin Oncol 2001; 20: 349a
Wheatley K, Hancock B, Gore M, et al. Interferon-a as adjuvant therapy for melanoma: a meta-analysis of the randomised trials [abstract]. Proc Am Soc Clin Oncol 2001; 20: 349a
Cameron DA, Cornbleet MC, Mackie RM, et al. Adjuvant interferon alpha 2b in high risk melanoma: the Scottish study. Br J Cancer 2001; 84(9): 1146–9
Cole BF, Gelber RD, Kirkwood JM, et al. Quality-of-life-adjusted survival analysis of interferon alfa-2b adjuvant treatment of high-risk resected cutaneous melanoma: an Eastern Cooperative Oncology Group study. J Clin Oncol 1996; 14(10): 2666–73
Agarwala SS, Kirkwood JM. Adjuvant interferon treatment for melanoma. Hematol Oncol Clin North Am 1998; 12(4): 823–33
Medical Education Network. Mediview Express Report: winners, losers and changes, highlight perspectives in melanoma conference. 4th Perspectives in Melanoma; 2000 Jun 1-2; Pittsburgh, 1-4
Kirkwood JM. Adjuvant therapy of high-risk resected melanoma: relapse-free and overall survival effects of high-dose interferon alpha-2b in randomized controlled multicenter trials El 684 and E2696 and intergroup trials El 690 and E1694. Proceedings of the 37th Annual Meeting of the American Society of Clinical Oncology; 2001 May 12-15; San Francisco, 94-101
Agarwala SS, Kirkwood JM. Update on the role of adjuvant interferon for high risk melanoma. FORUM Trends Exp Clin Med 2000; 10(3): 230–9
Eggermont AMM, Kleeberg UR, Ruiter DJ, et al. European Organization for the Research and Treatment of Cancer melanoma group trial experience with more than 2000 patients evaluating adjuvant treatment with low or intermediate doses of interferon alpha-2b. Proceedings of the 37th Annual Meeting American Society of Clinical Oncology; 2001 May 12-15; San Francisco, 88-93
Cascinelli N, Belli F, MacKie R, et al. Effect of long-term adjuvant therapy with interferon alpha-2a in patients with regional node metastases from cutaneous melanoma: a randomised trial. Lancet 2001; 358(9285): 866–9
Eggermont AMM. The role of interferon-alpha in malignant melanoma remains to be defined. Eur J Cancer 2001; 37: 2147–53
Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomised trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol 1999; 17(9): 2745–51
Houghton AN, Legha S, Bajorin DF. Chemotherapy for metastatic melanoma. 2nd ed. In: Balch CM, Houghton AN, Milton GW, et al., editors. Cutaneous melanoma. Philadelphia (PA): JB Lippincott, 1992: 499–508
Kirkwood JM, Ernstoff MS, Davis CA, et al. Comparison of intramuscular and intravenous recombinant alpha-2 interferon in melanoma and other cancers. Ann Intern Med 1985; 103(1): 32–6
Robinson WA, Mughal TI, Thomas MR, et al. Treatment of metastatic malignant melanoma with recombinant interferon alpha 2. Immunobiology 1986; 172(3-5): 275–82
Creagan ET, Ahman DL, Frytak S, et al. Three consecutive phase II studies of recombinant interferon alpha-2a in advanced malignant melanoma. Cancer 1987; 59 Suppl. 3: 638–46
Steiner A, Wolf C, Pehamberger H. Comparison of the effects of three different treatment regimens of recombinant interf erons (r-IFN alpha, r-IFN-gamma and r-IFN alpha + cimetidine) in disseminated malignant melanoma. J Cancer Res Clin Oncol 1987; 113: 459–65
Sertoli MR, Bernengo MG, Ardizzoni A, et al. Phase II trial of recombinant alpha-2b interferon in the treatment of metastatic skin melanoma. Oncology 1989; 46(2): 96–8
Sheridan E, Hancock BW. Systemic treatment of metastatic malignant melanoma. In: Kirkham N, Cotton DWK, Lallemand RC, et al., editors. Diagnosis and management of melanoma in clinical practice. London: Springer-Verlag, 1992: 135–56
de Takats PG, Williams MV, Hawkins R. Adjuvant therapy for melanoma: how should we respond to high-dose interferon? Cancer 1998; 77: 1287–93
Kirkwood JM. Melanoma. In: DeVita VTJ, Hellman S, Rosenberg SA, editors. Biologic therapy of cancer. 2nd ed. Philadelphia (PA): JB Lippincott, 1995: 388–411
Agarwala SS, Kirkwood JM. Interferon in melanoma. Curr Opin Oncol 1996; 8(2): 167–74
Kirkwood JM. Studies of interferons in the therapy of melanoma. Semin Oncol 1991; 18 5 Suppl. 7:83–90
Legha S. Current therapy for melanoma. Semin Oncol 1989; 16 1 Suppl. 1: 34–44
Creagan ET, Ahmann DL, Frytak S, et al. Phase II trials of recombinant leucocyte A interferon in disseminated malignant melanoma: results in 96 patients. Cancer Treat Rep 1986; 70(5): 619–24
Punt CJA. The use of interferon-α in the treatment of cutaneous melanoma: a review. Melanoma Res 1998; 8(2): 95–104
Hauschild A, Garbe C, Stolz W, et al. Dacarbazine and interferon-alpha with or without interleukin-2 in metastatic melanoma: a randomized phase III multicentre trial of the Dermatologic Cooperative Oncology Group (DeCOG). Br J Cancer 2001; 84(8): 1036–42
Keilholz U, Eggermont AMM, EORTC Melanoma Co-operative Group. The emerging role of cytokines in the treatment of advanced melanoma. Oncology 2000; 58(2): 89–95
Rosenberg SA, Lotze MT, Yang JC, et al. Combination therapy with interleukin-2 and alpha-interferon for the treatment of patients with advanced cancer. J Clin Oncol 1989; 7(12): 1863–74
Kruit WHJ, Punt CJA, Goey SH, et al. Dose efficacy study of two schedules of high-dose bolus administration of interleukin-2 and interferon alpha in metastatic melanoma. Br J Cancer 1996; 74(6): 951–5
Keilholz U, Scheibenbogen C, Tilgen W, et al. Interferon-a and interleukin-2 in the treatment of metastatic melanoma: comparison of two phase II trials. Cancer 1993; 72(2): 607–14
Sparano JA, Fisher RI, Sunderland M, et al. Randomised phase III trial of treatment with high-dose interleukin-2 either alone or in combination with interferon alfa-2a in patients with advanced melanoma. J Clin Oncol 1993; 11(10): 1969–77
Creagan ET, Loprinzi CL, Ahmann DL, et al. A phase I-II trial of recombinant leucocyte A interferon and recombinant interferon-gamma in patients with disseminated malignant melanoma. Cancer 1988; 62(12): 2472–4
Lee KH, Talpaz M, Rothberg JM, et al. Concomitant administration of recombinant human interleukin-2 and recombinant interferon alpha-2A in cancer patients: a phase I study. J Clin Oncol 1989; 7: 1726–32
Budd GT, Murthy S, Finke J, et al. Phase I trial of high-dose bolus interleukin-2 and interferon alfa-2a in patients with metastatic malignancy. J Clin Oncol 1992; 10: 804–9
Whitehead RP, Figlin R, Citron ML, et al. A phase II trial of concomitant interleukin-2 and interferon-alpha-2a in patients with disseminated malignant melanoma. J Immunother 1993; 13: 117–21
Kruit WHJ, Goey SH, Calabresi F, et al. Final report of a phase II study of interleukin 2 and interferon alpha in patients with metastatic melanoma. Br J Cancer 1995; 71: 1319–21
KhayatD, Coeffic D, Antoine E-C. Overview of medical treatments of metastatic malignant melanoma. Proceedings of the 36th Annual Meeting of the American Society of Clinical Oncology; 2000 May 20-23; New Orleans, 414-27
Falkson CI, Falkson G, Falkson HC. Improved results with the addition of interferon alfa-2b to dacarbazine in the treatment of patients with metastatic malignant melanoma. J Clin Oncol 1991; 9(8): 1403–8
Thomson DB, Adena M, McLeod GR, et al. Interferon-alpha 2a does not improve response or survival when combined with dacarbazine in metastatic malignant melanoma: results of a multi-institutional Australian randomized trial. Melanoma Res 1993; 3(2): 133–8
Bajetta E, Di Leo A, Zampino MG, et al. Multicenter randomized trial of dacarbazine alone or in combination with two different doses and schedules of interferon alfa-2a in the treatment of advanced melanoma. J Clin Oncol 1994; 12(4): 806–11
Falkson CI, Ibrahim J, Kirkwood JM, et al. Phase III trial of dacarbazine vs dacarbazine with interferon alpha2b vs dacarbazine with tamoxifen vs dacarbazine with interferon alpha2b with tamoxifen in patients with metastatic malignant melanoma. J Clin Oncol 1998; 16: 1743–51
Margolin KA, Doroshow JH, Akman SA, et al. Phase II trial of cisplatinum and alpha-interferon in advanced malignant melanoma. J Clin Oncol 1992; 10(10): 1574–8
Richner J, Joss RA, Goldhirsch A, et al. Phase II study of continuous subcutaneous interferon-alfa combined with cisplatin in advanced malignant melanoma. Eur J Cancer 1992; 28A(6/7): 1044–7
Pyrhonen S, Hahka-Kempinnen M, Muhonen T. A promising interferon plus four-drug chemotherapy regimen for metastatic melanoma. J Clin Oncol 1992; 10(12): 1919–26
Hahka-Kemppinen M, Muhonen T, Virolainen M, et al. Response of subcutaneous and cutaneous metastases of malignant melanoma to combined cytostatic plus interferon therapy. Br J Dermatol 1995; 132: 973–7
Vuoristo MS, Grohn P, Kellokumpu-Lehtinen P, et al. Intermittent interferon and polychemotherapy in metastatic melanoma. J Cancer Res Clin Oncol 1995; 121: 175–80
Stark JJ, Schulof R, Wiemann M, et al. Alpha interferon and chemo-hormonal therapy in advanced melanoma: a phase I-II NBSG/MAOP study [abstract]. Proc Am Soc Clin Oncol 1993; 12: 392a
Schultz M, Poo WJ, Buzaid AC. A phase II study of cisplatin (CDDP), dacarbazine (DTIC), carmustine (BCNU), tamoxifen (TM), and interferon-alpha 2B (alpha-rIFN) in metastatic melanoma [abstract]. Proc Am Soc Clin Oncol 1993; 12: 390a
Keilholz U, Scheibenbogen C, Stoelben E, et al. Immunotherapy of metastatic melanoma with interferon-alpha and interleukin-2: pattern of progression in responders and patients with stable disease with or without resection of residual lesions. Eur J Cancer 1994; 30A: 955–8
Legha S, Ring S, Bedikian A, et al. Lack of benefit from tamoxifen (T) added to a regimen of cisplatin (C), vinblastine (V), DTIC (D), and alfa interferon (rIFN) in patients (pts) with metastatic melanoma (MM) [abstract]. Proc Am Soc Clin Oncol 1993; 12: 388a
Mulder NH, deVries EGE, Sleijfer DT, et al. Dacarbazine (DTIC), human recombinant interferon alpha 2a (roferon) and 5-fluorouracil for disseminated malignant melanoma. Br J Cancer 1992; 65(2): 303–4
Punt CJA, van Herpen CML, Jansen RLH, et al. Chemoimmunotherapy with bleomycin, vincristine, lomustine, dacarbazine (BOLD) plus interferon-α for metastatic melanoma: a multicentre phase II study. Br J Cancer 1997; 76:266–9
Khayat D, Borel C, Tourani JM, et al. Sequential chemoimmunotherapy with cisplatin, interleukin-2 and interferon alfa-2a for metastatic melanoma. J Clin Oncol 1993; 11(11): 2173–80
Buzaid AC, Legha SS. Combination of chemotherapy with interleukin-2 and interferon-alfa for the treatment of advanced melanoma. Semin Oncol 1994; 21 6 Suppl. 14:23–8
Proebstle TM, Scheibenbogen C, Sterry W, et al. A phase II study of dacarbazine, cisplatin, interferon and high-dose interleukin-2 in ‘poor-risk’ metastatic melanoma. Eur J Cancer 1996; 32A(9): 1530–3
Legha SS, Bedikian A, Plager C, et al. Biochemotherapy studies in the treatment of advanced melanoma [abstract]. Melanoma Res 1997; 7 Suppl. 1: S24
Dorval T, Negrier S, Chevreau C, et al. Results of a French multicentric randomized trial of chemoimmunotherapy with or without interferon in metastatic malignant melanoma [abstract]. Proc Am Soc Clin Oncol 1994; 13: 1347a
Keilholz U, Goey SH, Punt CJ, et al. Interferon alfa-2a and interleukin-2 with or without cisplatinum in metastatic melanoma: a randomized trial of the European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Clin Oncol 1997; 15(7): 2579–88
Keilholz U, Punt C, Gore M, et al. Cisplatin and interferon alpha with or without interleukin-2 in advanced melanoma: interim analysis of EORTC 18951 [abstract]. Proc Am Soc Clin Oncol 1999; 18: 530a
Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Prospective randomized trial of the treatment of patients with metastatic melanoma using chemotherapy with cisplatin, dacarbazine, and tamoxifen alone or in combination with interleukin-2 and interferon alfa-2b. J Clin Oncol 1999; 17(3): 968–75
Eton O, Legha S, Bedikian A, et al. Phase III randomized trial of cisplatin, vinblastine and dacarbazine (CVD) plus interleukin-2 (IL-2) and interferon-alpha-2b (INF) vs CVD in patients (Pts) with metastatic melanoma [abstract]. Proc Am Soc Clin Oncol 2000; 19: 2174a
Creagan ET, Ahman DL, Green SJ, et al. Phase II study of recombinant leukocyte A interferon (IFN-rA) plus cimetidine in disseminated malignant melanoma. J Clin Oncol 1985; 3(7): 977–81
Creagan ET, Long HJ, Ahman DL, et al. Phase II assessment of recombinant leukocyte A interferon with difluoromethylornithine in disseminated malignant melanoma. Am J Clin Oncol 1990; 13(3): 218–20
Wadler S, Einzig AI, Dutcher JP, et al. Phase II trial of recombinant alpha-2b-interferon and low-dose cyclophosphamide in advanced melanoma and renal cell carcinoma. Am J Clin Oncol 1988; 11(1): 55–9
Dhingra K, Papadopoulos N, Lippman S, et al. Phase II study of alpha-interferon and 13-cisretinoic acid in metastatic melanoma. Invest New Drugs 1993; 11(1): 39–43
Rustin GJS, Dische S, De Garis ST, et al. Treatment of advanced malignant melanoma with interferon alpha and etretinate. Eur J Cancer Clin Oncol 1988; 24(4): 783–4
Miller RL, Steis RG, Clark JW, et al. Randomized trial of recombinant alpha 2b-interferon with or without indomethacin in patients with metastatic malignant melanoma. Cancer Res 1989; 49(7): 1871–6
Kirkwood JM, Agarwala SS, Diaz B, et al. Phase I study of temozolomide in combination with interferon alfa-2b in metastatic melanoma [abstract]. Proc Am Soc Clin Oncol 1997; 16: 491a
Hellsten S, Berge T, Wehlin L. Unrecognized renal cell carcinoma: clinical and pathological aspects. Scand J Urol Nephrol 1981; 15(3): 273–8
Hellsten S, Berge T, Wehlin L. Unrecognized renal cell carcinoma: clinical and diagnostic aspects. Scand J Urol Nephrol 1981; 15(3): 269–72
Oliver RT, Nethersell AB, Bottomley JM. Unexplained spontaneous regression and alpha-interferon as treatment for metastatic renal carcinoma. Br J Urol 1989; 63(2): 128–31
Vogelzang NJ, Priest ER, Borden L. Spontaneous regression of histologically proved pulmonary metastases from renal cell carcinoma: a case with 5-year followup. J Urol 1992; 148(4): 1247–8
Tykka H, Oravisto KJ, Lehtonen T, et al. Active specific immunotherapy of advanced renal-cell carcinoma. Eur Urol 1978; 4(4): 250–8
Quesada JR, Swanson DA, Trindade A, et al. Renal cell carcinoma: antitumor effects of leukocyte interferon. Cancer Res 1983; 43(2): 940–7
Nanus DM, Pfeffer LM, Bander NH, et al. Antiproliferative and antitumor effects of alpha-interferon in renal cell carcinomas: correlation with the expression of a kidney-associated differentiation glycoprotein. Cancer Res 1990; 50(14): 4190–4
Kuebler JP, Oberley TD, Meisner LF, et al. Effect of interferon alpha, interferon beta, and interferon gamma on the in vitro growth of human renal adenocarcinoma cells. Invest New Drugs 1987; 5(1): 21–9
Hernberg M, Pyrhonen S, Muhonen T. Regimens with or without interferon-alpha as treatment for metastatic melanoma and renal cell carcinoma: an overview of randomized trials. J Immunother 1999; 22(2): 145–54
Porzsolt F, Messerer D, Hautmann R, et al. Treatment of advanced renal cell cancer with recombinant interferon alpha as a single agent and in combination with medroxyprogesterone acetate: a randomized multicenter trial. J Cancer Res Clin Oncol 1988; 114(1): 95–100
Quesada JR, Rios A, Swanson D, et al. Antitumor activity of recombinant-derived interferon alpha in metastatic renal cell carcinoma. J Clin Oncol 1985; 3(11): 1522–8
Kirkwood JM, Harris JE, Vera R, et al. A randomized study of low and high doses of leukocyte alpha-interferon in metastatic renal cell carcinoma: the American Cancer Society collaborative trial. Cancer Res 1985; 45(2): 863–71
Steineck G, Strander H, Carbin BE, et al. Recombinant leukocyte interferon alpha-2a and medroxyprogesterone in advanced renal cell carcinoma: a randomized trial. Acta Oncol 1990; 29(2): 155–62
deKernion JB, Sarna G, Figlin R, et al. The treatment of renal cell carcinoma with human leukocyte alpha-interferon. J Urol 1983; 130(6): 1063–6
Quesada JR, Swanson DA, Gutterman JU. Phase II study of interferon alpha in metastatic renal-cell carcinoma: a progress report. J Clin Oncol 1985; 3(8): 1086–92
Umeda T, Niijima T. Phase II study of alpha interferon on renal cell carcinoma: summary of three collaborative trials. Cancer 1986; 58(6): 1231–5
Kempf RA, Grunberg SM, Daniels JR, et al. Recombinant interferon alpha-2 (Intron A) in a phase II study of renal cell carcinoma. J Biol Response Mod 1986; 5(1): 27–35
Muss HB, Costanzi JJ, Leavitt R, et al. Recombinant alfa interferon in renal cell carcinoma: a randomized trial of two routes of administration. J Clin Oncol 1987; 5(2): 286–91
Trump DL, Elson PJ, Borden EC, et al. High-dose lymphoblastoid interferon in advanced renal cell carcinoma: an Eastern Cooperative Oncology Group Study. Cancer Treat Rep 1987; 71(2): 165–9
Minasian LM, Motzer RJ, Gluck L, et al. Interferon alfa-2a in advanced renal cell carcinoma: treatment results and survival in 159 patients with long-term follow-up. J Clin Oncol 1993; 11(7): 1368–75
Medical Research Council Renal Cancer Collaborators. Interferon-alpha and survival in metastatic renal carcinoma: early results of a randomised controlled trial. Lancet 1999; 353(9146): 14–7
Hancock B, Griffiths G, Ritchie A, et al. Updated results of the MRC randomised controlled trial of alpha interferon vs MPA in patients with metastatic renal cell carcinoma [abstract]. Proc Am Soc Clin Oncol 2000; 19: 340a
Nieforth KA, Nadeau R, Indravadan H, et al. Use of an indirect pharmacodynamic stimulation model of MX protein induction to compare in vivo activity of interferon alfa-2a and a polyethylene glycol-modified derivative in healthy subjects. Clin Pharmacol Ther 1996; 59(6): 636–46
Motzer RJ, Thompson J, Gurney H, et al. Phase II trial of branched pegylated (40 kDa) interferon alpha 2a for patients with advanced renal cell carcinoma [abstract]. Proc Am Soc Clin Oncol 2001; 20: 180a
Figlin RA, Belldegrun A, Moldawer N, et al. Concomitant administration of recombinant human interleukin-2 and recombinant interferon alfa-2A: an active outpatient regimen in metastatic renal cell carcinoma. J Clin Oncol 1992; 10(3): 414–21
Ratain MJ, Priest ER, Janisch L, et al. A phase I study of subcutaneous recombinant interleukin-2 and interferon alfa-2a. Cancer 1993; 71(7): 2371–6
Vogelzang NJ, Lipton A, Figlin RA. Subcutaneous interleukin-2 plus interferon alfa-2a in metastatic renal cancer: an outpatient multicenter trial. J Clin Oncol 1993; 11(9): 1809–16
Ravaud A, Negrier S, Cany L, et al. Subcutaneous low-dose recombinant interleukin 2 and alpha-interferon in patients with metastatic renal cell carcinoma. Br J Cancer 1994; 69(6): 1111–4
Passalacqua R, Caminiti C, Cengarle R, et al. Efficacy of a regimen of chronic low dose of interleukin 2 (IL-2) and Interferon alfa (IFN) in metastatic renal cell carcinoma [abstract]. Proc Am Soc Clin Oncol 2001; 20: 195a
Lissoni P, Barni S, Ardizzoia A, et al. A randomized study of low-dose interleukin-2 subcutaneous immunotherapy vs interleukin-2 plus interferon-alpha as first line therapy for metastatic renal cell carcinoma. Tumori 1993; 79(6): 397–400
Boccardo F, Rubagotti A, Canobbio L, et al. Interleukin-2, interferon-alpha and interleukin-2 plus interferon-alpha in renal cell carcinoma: a randomized phase II trial. Tumori 1998; 84(5): 534–9
Henriksson R, Nilsson S, Colleen S, et al. Survival in renal cell carcinoma -a randomized evaluation of tamoxifen vs interleukin 2, alpha-interferon (leucocyte) and tamoxifen. Br J Cancer 1998; 77(8): 1311–7
Jayson GC, Middleton M, Lee SM, et al. A randomized phase II trial of interleukin 2 and interleukin 2-interferon alpha in advanced renal cancer. Br J Cancer 1998; 78(3): 366–9
Negrier S, Escudier B, Lasset C, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d’Immunotherapie. N Engl J Med 1998; 338(18): 1272–8
Mcdermott D, Flaherty L, Clark J. A randomised phase III trial of high dose interleukin-2 (HD IL2) vs subcutaneous IL-2 /interferon (IFN) in patients with metastatic renal cell carcinoma [abstract]. Proc Am Soc Clin Oncol 2001; 20:172a
Atzpodien J, Kirchner H, Hanninen EL, et al. Interleukin-2 in combination with interferon-alpha and 5-fluorouracil for metastatic renal cell cancer. Eur J Cancer 1993; 29A Suppl. 5: S6–8
Hofmockel G, Langer W, Theiss M, et al. Immunochemotherapy for metastatic renal cell carcinoma using a regimen of interleukin-2, interferon-alpha and 5-fluorouracil. J Urol 1996; 156(1): 18–21
Joffe JK, Banks RE, Forbes MA, et al. A phase II study of interferon-alpha, interleukin-2 and 5-fluorouracil in advanced renal carcinoma: clinical data and laboratory evidence of protease activation. Br J Urol 1996; 77(5): 638–49
Lopez Hanninen E, Kirchner H, Atzpodien J. Interleukin-2 based home therapy of metastatic renal cell carcinoma: risks and benefits in 215 consecutive single institution patients. J Urol 1996; 155(1): 19–25
Ellerhorst JA, Sella A, Amato RJ, et al. Phase II trial of 5-fluorouracil, interferon-alpha and continuous infusion interleukin-2 for patients with metastatic renal cell carcinoma. Cancer 1997; 80(11): 2128–32
Ravaud A, Audhuy B, Gomez F, et al. Subcutaneous interleukin-2, interferon alfa-2a, and continuous infusion of fluorouracil in metastatic renal cell carcinoma: a multicenter phase II trial. Groupe Francais d’Immunotherapie. J Clin Oncol 1998; 16(8): 2728–32
Elias L, Binder M, Mangalik A, et al. Pilot trial of infusional 5-fluorouracil, interleukin-2, and subcutaneous interferon-alpha for advanced renal cell carcinoma. Am J Clin Oncol 1999; 22(2): 156–61
Samland D, Steinbach F, Reiher F, et al. Results of immunochemotherapy with interleukin-2, interferon-alpha2 and 5-fluorouracil in the treatment of metastatic renal cell cancer. Eur Urol 1999; 35(3): 204–9
Allen MJ, Vaughan M, Webb A, et al. Protracted venous infusion 5-fluorouracil in combination with subcutaneous interleukin-2 and alpha-interferon in patients with metastatic renal cell cancer: a phase II study. Br J Cancer 2000; 83(8): 980–5
Dutcher JP, Logan T, Gordon M, et al. Phase II trial of interleukin 2, interferon alpha, and 5-fluorouracil in metastatic renal cell cancer: a cytokine working group study. Clin Cancer Res 2000; 6(9): 3442–50
Elias L, Lew D, Figlin RA, et al. Infusional interleukin-2 and 5-fluorouracil with subcutaneous interferon-alpha for the treatment of patients with advanced renal cell carcinoma: a Southwest Oncology Group phase II study. Cancer 2000; 89(3): 597–603
vanHerpen CM, Jansen RL, Kruit WH, et al. Immunochemotherapy with interleukin-2, interferon-alpha and 5-fluorouracil for progressive metastatic renal cell carcinoma: a multicenter phase II study. Dutch Immunotherapy Working Party. Br J Cancer 2000; 82(4): 772–6
Negrier S, Caty A, Lesimple T, et al. Treatment of patients with metastatic renal carcinoma with a combination of subcutaneous interleukin-2 and interferon alfa with or without fluorouracil. Groupe Francais d’Immunotherapie, Federation Nationale des Centres de Lutte contre le Cancer. J Clin Oncol 2000; 18(24): 4009–15
Pectasides D, Varthalitis J, Kostopoulou M, et al. An outpatient phase II study of subcutaneous interleukin-2 and interferon-alpha-2b in combination with intravenous vinblastine in metastatic renal cell cancer. Oncology 1998; 55(1): 10–5
Fossa SD, Martinelli G, Otto U, et al. Recombinant interferon alfa-2a with or without vinblastine in metastatic renal cell carcinoma: results of a European multi-center phase III study. Ann Oncol 1992; 3(4): 301–5
Pyrhonen S, Salminen E, Ruutu M, et al. Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol 1999; 17(9): 2859–67
Sertoli MR, Brunetti I, Ardizzoni A, et al. Recombinant alpha-2a interferon plus vinblastine in the treatment of metastatic renal cell carcinoma. Am J Clin Oncol 1989; 12(1): 43–5
De Mulder PH, Oosterhof G, Bouffioux C, et al. EORTC (30885) randomised phase III study with recombinant interferon alpha and recombinant interferon alpha and gamma in patients with advanced renal cell carcinoma. The EORTC Genitourinary Group. Br J Cancer 1995; 71(2): 371–5
Sagaster P, Micksche M, Flamm J, et al. Randomised study using IFN-alpha versus IFN-alpha plus coumarin and cimetidine for treatment of advanced renal cell cancer. Ann Oncol 1995; 6(10): 999–1003
Creagan ET, Twito DI, Johansson SL, et al. A randomized prospective assessment of recombinant leukocyte A human interferon with or without aspirin in advanced renal adenocarcinoma. J Clin Oncol 1991; 9(12): 2104–9
Gebrosky NP, Koukol S, Nseyo UO, et al. Treatment of renal cell carcinoma with 5-fluorouracil and alfa-interferon. Urology 1997; 50(6): 863–7; discussion 867-8
Stadler WM, Kuzel T, Dumas M, et al. Multicenter phase II trial of interleukin-2, interferon-alpha, and 13-cis-retinoic acid in patients with metastatic renal-cell carcinoma. J Clin Oncol 1998; 16(5): 1820–5
Wilding G, Carducci MA, Hwu W, et al. A multicentre phase I/II evaluation of oral bexarotene (BEX) and interferon alfa 2b combination therapy in patients with advanced renal cell carcinoma [abstract]. Proc Am Soc Clin Oncol 2001; 20: 191a
Donskov F, Von der Masse H, Henriksson R, et al. Multicentre phase II study with the combination of histamine dihydrochloride, interferon alfa and interleukin-2 in patients with metastatic renal cell carcinoma [abstract]. Proc Am Soc Clin Oncol 2001; 20: 264a
Fossa SD, Aune H, Baggerud E, et al. Continuous intravenous interleukin-2 infusion and subcutaneous interferon-alpha in metastatic renal cell carcinoma. Eur J Cancer 1993; 29A(9): 1313–5
Palmer PA, Atzpodien J, Philip T, et al. A comparison of 2 modes of administration of recombinant interleukin-2: continuous intravenous infusion alone vs subcutaneous administration plus interferon alpha in patients with advanced renal cell carcinoma. Cancer Biother 1993; 8(2): 123–36
Thiounn N, Mathiot C, Dorval T, et al. Lack of efficacy of low-dose subcutaneous recombinant interleukin-2 and interferon-alpha in the treatment of metastatic renal cell carcinoma. Br J Urol 1995; 75(5): 586–9
Rogers E, Bredin H, Butler M, et al. Combined subcutaneous recombinant alpha-interferon and interleukin-2 in metastatic renal cell cancer: results of the multicentre All-Ireland Immunotherapy Study Group. Eur Urol 2000; 37(3): 261–6
Tourani JM, Pfister C, Berdah JF. Subcutaneous interleukin-2 plus alpha interferon in out-patients with metastatic renal cell carcinoma. SCAPP III trial [abstract]. Proc Am Soc Clin Oncol 2000; 19: 334a
Tourani JM, Pfister C, Berdah JF, et al. Outpatient treatment with subcutaneous interleukin-2 and interferon alfa administration in combination with fluorouracil in patients with metastatic renal cell carcinoma: results of a sequential nonrandomized phase II study. Subcutaneous administration Propeukin Program Cooperative Group. J Clin Oncol 1998; 16(7): 2505–13
Olencki T, Peereboom D, Wood L, et al. Phase I and II trials of subcutaneously administered rIL-2, interferon alfa-2a, and fluorouracil in patients with metastatic renal carcinoma. J Cancer Res Clin Oncol 2001; 127(5): 319–24
Massidda B, Migliari R, Padovani A, et al. Metastatic renal cell cancer treated with recombinant alpha 2a interferon and vinblastine. J Chemother 1991; 3(6): 387–9
Cetto GL, Franceschi T, Turrina G, et al. Recombinant alpha-interferon and vinblastine in metastatic renal cell carcinoma: efficacy of low doses. Semin Surg Oncol 1988; 4(3): 184–90
Fossa SD, Raabe N, Moe B. Recombinant interferon-alpha with or without vinblastine in metastatic renal carcinoma: results of a randomised phase II study. Br J Urol 1989; 64(5): 468–71
Kriegmair M, Oberneder R, Hofstetter A. Interferon alfa and vinblastine vs medroxyprogesterone acetate in the treatment of metastatic renal cell carcinoma. Urology 1995; 45(5): 758–62
Neidhart JA, Anderson SA, Harris JE, et al. Vinblastine fails to improve response of renal cancer to interferon alfa-n1: high response rate in patients with pulmonary metastases. J Clin Oncol 1991; 9(5): 832–6
Nanus DM, Geng Y, Shen R, et al. Interaction of retinoic acid and interferon in renal cancer cell lines. J Interferon Cytokine Res 2000; 20(9): 787–94
Motzer RJ, Schwartz L, Law TM, et al. Interferon alfa-2a and 13-cis-retinoic acid in renal cell carcinoma: antitumor activity in a phase II trial and interactions in vitro. J Clin Oncol 1995; 13(8): 1950–7
Motzer RJ, Murphy BA, Bacik J, et al. Phase III trial of interferon alfa-2a with or without 13-cis-retinoic acid for patients with advanced renal cell carcinoma. J Clin Oncol 2000; 18(16): 2972–80
Vaishampayan U, Flaherty L, Du W, et al. Phase II evaluation of paclitaxel, alpha-interferon, and cis-retinoic acid in advanced renal cell carcinoma. Cancer 2001; 92(3): 519–23
Koga S, Nishikido M, Matsuya F, et al. Subcutaneous administration of interferon alpha and gamma in patients with metastatic renal cell carcinoma. Anticancer Res 1999; 19(6C): 5547–50
Foon K, Doroshow J, Bonnem E, et al. A prospective randomized trial of alpha 2B-interferon/gamma-interferon or the combination in advanced metastatic renal cell carcinoma. J Biol Response Mod 1988; 7(6): 540–5
Igarashi T, Marumo K, Onishi T, et al. Interferon-alpha and 5-fluorouracil therapy in patients with metastatic renal cell cancer: an open multicenter trial. The Japanese Study Group Against Renal Cancer. Urology 1999; 53(1): 53–9
Sparano JA, Wadler S, Diasio RB, et al. Phase I trial of low-dose, prolonged continuous infusion fluorouracil plus interferon-alfa: evidence for enhanced fluorouracil toxicity without pharmacokinetic perturbation. J Clin Oncol 1993; 11(8): 1609–17
Henriksson R. Survival in renal cell carcinoma: a randomized evaluation of tamoxifen vs interleukin-2, alpha-interferon (leucocyte) and tamoxifen -reply. Br J Cancer 2000; 82(1): 246–7
Kankuri M, Pelliniemi TT, Pyrhonen S, et al. Feasibility of prolonged use of interferon-alpha in metastatic kidney carcinoma: a phase II study. Cancer 2001; 92(4): 761–7
Fossa S, Jones M, Johnson P, et al. Interferon-alpha and survival in renal cell cancer. Br J Urol 1995; 76(3): 286–90
Creagan ET, Veeder MH, Suman VJ, et al. A phase II study of high-dose cimetidine and the combination 5-fluorouracil, interferon alpha-2A, and leucovorin in advanced renal cell adenocarcinoma. Am J Clin Oncol 1998; 21(5): 475–8
Buzio C, Andrulli S, Santi R, et al. Long-term immunotherapy with low-dose interleukin-2 and interferon-alpha in the treatment of patients with advanced renal cell carcinoma. Cancer 2001; 92(9): 2286–96
Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med 1996; 335(12): 865–75
Phillips E, Messing EM. Role of lymphadenectomy in the treatment of renal cell carcinoma. Urology 1993; 41(1): 9–15
Rabinovitch RA, Zelefsky MJ, Gaynor JJ, et al. Patterns of failure following surgical resection of renal cell carcinoma: implications for adjuvant local and systemic therapy. J Clin Oncol 1994; 12(1): 206–12
Sandock DS, Seftel AD, Resnick MI. A new protocol for the followup of renal cell carcinoma based on pathological stage. J Urol 1995; 154(1): 28–31
Takahashi S, Tanigawa T, Imagawa M, et al. Interferon as adjunctive treatment for non-metastatic renal cell carcinoma. Br J Urol 1994; 74(1): 11–4
Trump DL, Elson P, Propert K. Randomised controlled trial of adjuvant therapy with lymphoblastoid interferon (L-IFN) in resected, high risk renal cell carcinoma [abstract]. Proc Am Soc Clin Oncol 1996; 15: 253a
Pizzocaro G, Piva L, Colavita M, et al. Interferon adjuvant to radical nephrectomy in Robson stages II and III renal cell carcinoma: a multicentric randomized study. J Clin Oncol 2001; 19(2): 425–31
Porzsolt F. Adjuvant therapy of renal cell cancer with interferon alfa-2a [abstract]. Proc Am Soc Clin Oncol 1992; 11: 202a
Prummer O. Interferon-alpha antibodies in patients with renal cell carcinoma treated with recombinant interferon-alpha-2A in an adjuvant multicenter trial. The Delta-P Study Group. Cancer 1993; 71(5): 1828–34
Kirkwood JM, Manola J, Ibrahim JG, et al. Pooled analysis of four ECOG-intergroup trials of high-dose interferon alfa-2b (HDI) in 1916 patients with high-risk resected cutaneous melanoma [abstract]. Proc Am Soc Clin Oncol 2001; 20:1395
Russell-Jones R. Interferon-alpha therapy for melanoma. Clin Exp Dermatol 2000; 25(1): 1–6
Lens MB, Dawes M. Interferon alfa therapy for malignant melanoma: a systematic review of randomized controlled trials. J Clin Oncol 2002; 20(7): 1818–25
Gray RJ, Pockaj BA, Kirkwood JM. An update on adjuvant interferon for melanoma. Cancer Control 2002; 9(1): 16–21
Zoller M. IFN-treatment of B166-F1 versus B16-F10: Relative impact on nonadaptive and T-cell mediated immune defense in metastatic spread. Clin Exp Metastasis 1988; 6(5): 411–29
Keilholz U, Scheibenbogen C, Sommer M, et al. Prognostic factors for response and survival in patients with metastatic melanoma receiving immunotherapy. Melanoma Res 1996; 6(2): 173–8
Allen REJ, Epstein WL. Melanoma therapy. Clin Dermatol 1992; 10: 317–25
Maral J. Long term follow up of patients with metastatic malignant melanoma treated continuous infusion-IL2 alone or in combination. 8th International Congress Anticancer Treatment Chiron Symposium; 1998 Feb 3-6; Paris. Paris: SOMPS, 1998
Schrader AJ. Combined chemoimmunotherapy in metastatic melanoma: is there a need for the double? Anticancer Drugs 2000; 11(3): 143–8
Motzer RJ, Mazumdar M, Bacik J, et al. Effect of cytokine therapy on survival for patients with advanced renal cell carcinoma. J Clin Oncol 2000; 18(9): 1928–35
Uygur MC, Usubutun A, Ozen H, et al. Prognostic factors and the role of nephrectomy in metastatic renal cell carcinoma. J Exp Clin Cancer Res 1999; 18(3): 397–401
Fossa SD, Kramar A, Droz JP. Prognostic factors and survival in patients with metastatic renal cell carcinoma treated with chemotherapy or interferon-alpha. Eur J Cancer 1994; 30A(9): 1310–4
Flanigan RC, Blumenstein BA, Salmon S, et al. Cytoreductive tumor nephrectomy in metastatic renal cancer: the results of Southwest Oncology Group trial 8949 [abstract]. J Urol 2000; 163 Suppl.: 154
Mickisch GHJ, Garin A, van Poppel H, et al. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal cell carcinoma: a randomised trial. Lancet 2001; 358(9286): 966–70
Klotz L. Back to nephrectomy for patients with metastatic renal cell cancer. Lancet 2001; 358(9286): 948–9
Santhanam S, Decatris M, O’Byrne K. Potential of interferon-α in solid tumours: part 2. Biodrugs. In press
Acknowledgements
M. Decatris and S. Santhanam contributed equally to this work. No sources of funding were used to assist in the preparation of this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Decatris, M., Santhanam, S. & O’Byrne, K. Potential of Interferon-α in Solid Tumours. BioDrugs 16, 261–281 (2002). https://doi.org/10.2165/00063030-200216040-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-200216040-00003