Abstract
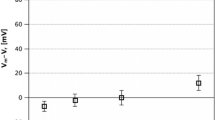
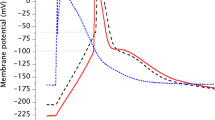
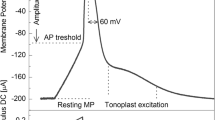
The role of acetylcholine (ACh) as a signalling molecule in plants was investigated using a model system of Characeae cells. The effect of ACh on conductance of K+ channels in Nitella flexilis cells and on the action potential generation in Nitellopsis obtusa cells after H+-ATPase inhibition, where repolarization occurs after the opening of outward rectifying K+ channels, was investigated. Voltage-clamp method based on only one electrode impalement was used to evaluate the activity of separate potassium ion transport system at rest. We found that ACh at high concentrations (1 mM and 5 mM) activates K+ channels as the main membrane transport system at the resting state involved in electrogenesis of Characeaen membrane potential. We observed that ACh caused an increase in duration of AP repolarization of cells in K+ state when plasmalemma electrical characteristics are determined by large conductance K+ channels irrespective of whether AP were spontaneous or electrically evoked. These results indicate interference of ACh with electrical cellular signalling pathway in plants.
Similar content being viewed by others
References
Wessler I., Kirkpatrick C.J., Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans, Br. J. Pharmacol., 2008, 154, 1558–1571
Resende R.R., Adhikari A., Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation, Cell Commun. Signal., 2009, 7–20
Arias H.R., Richards V.E., Ng D., Ghafoori M.E., Le V., Mousa S.A., Role of non-neuronal nicotinic acetylcholine receptors in angiogenesis, Int. J. Biochem. Cell Biol., 2009, 41, 1441–1451
Rodriguez-Diaz R., Dando R., Jacques-Silva M.C., Fachado A., Molina J., Abdulreda M.H., et al., Alpha cells secrete acetylcholine as a nonneuronal paracrine signal priming beta cell function in humans, Nat. Med., 2011, 17, 888–892
Roshchina V.V., Neurotransmitters in plant life, Science publishers, USA, 2001
Wessler I., Kilbinger H., Bittinger F., Kirkpatrick C.J., The biological role of non-neuronal acetylcholine in plants and humans, Jpn. J. Pharmacol., 2001, 85, 2–10
Hartmann E., Gupta R., Acetylcholine as a signaling system in plants, In: Boss W.E., Marre, D.J. Liss A.R. (Eds.), Second Messengers in Plant Growth and Development, Oxford, 1989
Horiuchi Y., Kimura R., Kato N., Fujii T., Seki M., Endo T., et al., Evolutional study on acetylcholine expression, Life Sci., 2003, 72, 1745–1756
Davies E., New functions for electrical signals in plants, New Phytologist, 2004, 161, 607–610
Knight H., Knight M. R., Abiotic stress signalling pathways: specificity and cross-talk, Trends Plant Sci., 2001, 6, 262–267
Sanders D., Brownlee C., Harper J. F., Communicating with calcium, Plant Cell, 1999, 11, 691–706
Zimmermann S., Ehrhardt T., Plesch G., Mueller-Roeber B., Ion channels in plant signaling, Cell Mol. Life Sci., 1999, 55, 183–203
Beilby M. J., Action potential in Charophytes, Int. Rev. Cytol., 2007, 257, 43–82
Hille B., Ion Channels of Excitable Membranes, 3rd ed, Sinauer Associates Inc., Sunderland, MA, 2001
Sondergaard T.E., Schulz A., Palmgren M.G., Energization of transport processes in plants. Roles of the plasma membrane H+-ATPase, Plant Physiol., 2004, 136, 2475–2482
Zingarelli L., Marre M.T., Massardi F., Lado P., Effects of hyper-osmotic stress on K+ fluxes, H+ extrusion, transmembrane electric potential difference and comparison with the effects of fusicoccin, Physiol. Plant, 1999, 106, 287–295
Sukhov V., Nerush V., Orlova L., Vodeneev V., Simulation of action potential propagation in plants, J. Theor. Biol., 2011, 291, 47–55
Kishimoto U., Takeuchi Y., Ohkawa T.A., Kami-ike N., A kinetic analysis of the electrogenic pump of Chara corallina: III. Pump activity during the action potential, J. Membr. Biol., 1985, 86, 27–36
Sukhov V.S., Vodeneev V.A., A mathematical model of action potential in cells of vascular plants, J. Membr. Biol., 2009, 232, 59–67
Johnson B.R., Wyttenbach R.A., Wayne R., Hoy R.R., Action potentials in a giant algal cell: a comparative approach to mechanisms and evolution of excitability, J. Undergrad. Neurosci. Educ., 2002, 1, 23–27
Thiel G., Homann U., Plieth C., Ion channel activity during the action potential in Chara: a new insight with new techniques, J. Exp. Bot., 1997, 48, 609–622
Gong X.-Q., Bisson M.A., Acetylcholine-activated Cl− channel in the Chara tonoplast, J. Membr. Biol., 2002, 188, 107–113
Volkov A.G., Plant electrophysiology — Theory and Methods, Springer-Verlag, Berlin, 2006
Sokolik A.I., Yurin V.M., Potasium channels in plasmalema of Nitella cells at rest, J. Membr. Biol., 1986, 89, 9–22
Yurin V.M., Sokolik A.I., Kudryashov A.P., Regulation of ion transport through plant cell membranes, Science and Engineering, Minsk, 1991
Kisnieriene V., Sakalauskas V., The effect of aluminium on bioelectrical activity of the Nitellopsis obtusa cell membrane after H+-ATPase inhibition, Cent. Eur. J. Biol., 2007, 2, 222–232
Beilby M.J., Shepherd V.A., The characteristics of Ca2+-activated Cl− channels of the salt-tolerant Charophyte Lamprothamnium, Plant Cell Environ., 2006, 29, 764–777
Jaffe M.J., Evidence for the regulation of phytochrome-mediated process in bean roots by the neurohumor, acetylcholine, Plant Physiol., 1970, 46, 768–777
Tretyn A., Influence of red light and acetylcholine on 45Ca2+ uptake by oat coleoptile cells, Cell Biol. Int. Rep., 1987, 11, 887–896
Lunevsky V.Z., Zherelova O.M., Vostrikov I.Y., Berestovsky G.N., Excitation of Characeae cell membranes as a result of activation of calcium and chloride channels, J. Membr. Biol., 1983, 72, 43–58
Kisnierienė V., Sakalauskas V., Pleskačiauskas A., Yurin V., Rukšėnas O., The combined effect of Cd2+ and ACh on action potentials of Nitellopsis obtusa cells, Cent. Eur. J. Biol., 2009, 4, 343–350
Tsutsui I., Ohkawa T., Regulation of the H+ pump activity in the plasma membrane of internally perfused Chara coralline, Plant Cell Physiol., 2001, 42, 531–537
Hirschi K., Vacuolar H+/Ca2+ transport: who’s directing the traffic?, Trends Plant Sci., 2001, 6, 100–104
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kisnieriene, V., Ditchenko, T.I., Kudryashov, A.P. et al. The effect of acetylcholine on Characeae K+ channels at rest and during action potential generation. cent.eur.j.biol. 7, 1066–1075 (2012). https://doi.org/10.2478/s11535-012-0085-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11535-012-0085-5