Abstract
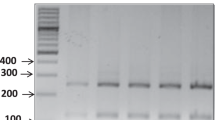
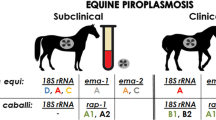
This study aimed to determine whether asymptomatic horses naturally infected with Theileria equi retain infected erythrocytes in the spleen and whether the presence of the hemoparasite in this organ is associated with parasitemia. We collected samples from 25 adult horses without clinical signs of any disease. From each animal, we collected whole blood samples from the jugular vein and a splenic puncture blood sample. All samples were submited to blood cell counts and detection of Theileria or Babesia. DNA extraction and PCR were performed in all samples for identification of piroplasm infection (T. equi and B. caballi). From the 25 horses evaluated for piroplasm detection by PCR, seven horses (28%) were positive in jugular vein blood but negative in splenic blood samples, five horses (20%) were positive in splenic blood samples but negative in jugular vein blood samples, and 13 horses (52%) were positive in both jugular vein and splenic blood samples. The hematological evaluation revealed anemia in 13 of 25 (52%) infected horses, lymphopenia in five (20%), neutrophilia in two (8%), neutropenia in one (4%), and thrombocytopenia in one (4%) infected horse. The present study demonstrated that several (20%) of the asymptomatic piroplasm carrier horses did not show parasitemia, but show infected erythrocytes in the spleen.
Similar content being viewed by others
References
Abutarbush S.M., Alqawasmeh D.M., Mukbel R.M., Al-Majali A.M. 2012. Equine babesiosis: seroprevalence, risk factors and comparison of different diagnostic methods in Jordan. Transboundary and Emerging Diseases, 59, 72–78. DOI: 10.1111/j.1865-1682.2011.01244.x.
Alhassan A., Govind Y., Tam N.T., Thekisoe O.M.M., Yokoyama N., Inoue N., Igarashi I. 2007a. Comparative evaluation of the sensitivity of LAMP, PCR and in vitro culture methods for the diagnosis of equine piroplasmosis. Parasitology Research, 100, 1165–1168. DOI: DOI: 10.1007/s00436-006-0430-6.
Alhassan A., Iseki H., Kim C., Yokoyama N., Igarashi I. 2007b. Comparison of polymerase chain reaction methods for the detection of Theileria equi infection using whole blood compared with pre-extracted DNA samples as PCR templates. Tropical Animal Health and Production, 39, 369–374. DOI: 10.1007/s11250-007-9025-1.
Alhassan A., Pumidonming W., Okamura M., Hirata H., Battsetseg B., Fujisaki K., Yokoyama N., Igarashi I. 2005. Development of a single-round and multiplex PCR method for the simultaneous detection of Babesia caballi and Babesia equi in horse blood. Veterinary Parasitology, 129, 43–49. DOI: 10.1016/j.vetpar.2004.12.018.
Allsopp M.T.E.P., Cavalier-Smith T., De Waal D.T., Allsopp B.A. 1994. Phylogeny and evolution of the piroplasms. Parasitology, 108, 147–152. DOI: 10.1017/S0031182000068232.
Bashiruddin J., Camma C., Rebelo E. 1999. Molecular detection of Babesia equi and Babesia caballi in horse blood by PCR amplification of part of the 16S rRNA gene. Veterinary Parasitology, 84, 75–83. DOI: 10.1016/S0304-4017(99)00049-7.
Bhoora R., Franssen L., Oosthuizen M.C., Guthrie A.J., Zweygarth E., Penzhorn B.L., Jongejan F., Collins N.E. 2009. Sequence heterogeneity in the 18S rRNA gene within Theileria equi and Babesia caballi from horses in South Africa. Veterinary Parasitology, 159, 112–120. DOI: 10.1016/j.vetpar.2008.10.004.
Böse R., Jorgensen W.K., Dalgliesh R.J., Friedhoff K.T., deVos A.J. 1995. Current state and future trends in the diagnosis of babesiosis. Veterinary Parasitology, 57, 61–74. DOI: 10.1016/0304-4017(94)03111-9.
Botteon P.T.L., Botteon R.C.C.M., Reis T.P., Massard C.L. 2005. Babesiose em cavalos atletas portadores. Ciência Animal, 35, 1136–1140.
Bruning E. 1996. Equine piroplasmosis: An update on diagnosis, treatment, and prevention. British Veterinary Journal, 152, 139–151. DOI: 10.1016/S0007-1935(96)80070-4.
Bulling A., Criado-Fornelio A., Asenzo G., Benitez D., Barba-Carretero J.C., Florin-Christensen M. 2007. A quantitative PCR assay for the detection and quantification of Babesia bovis and B. bigemina. Veterinary Parasitology, 147, 16–25. DOI:10.1016/j.vetpar.2007.03.031.
Carcy B., Précigout E., Schetters T., Gorenflot A. 2006. Genetic basis for GPI-anchor merozoite surface antigen polymorphism of Babesia and resulting antigenic diversity. Veterinary Parasitology, 138, 33–49. DOI: 10.1016/j.vetpar.2006.01.038.
Costa V.M.M., Rodrigues A.L., Medeiros J.M.A., Labruna M.B., Simões S.V.D., Riet-Correa F.2011. Tristeza parasitária bovina no Sertão da Paraíba. Pesquisa Veterinária Brasileira, 31, 239–243 DOI: 10.1590/S0100-736X2011000300009.
Criado-Fornelio A., Gónzalez-del-Río M.A., Buling-Saraña A., Barba-Carretero J.C. 2004. The “expanding universe” of piroplasms. Veterinary Parasitology, 119, 337–345. DOI:10.1016/j.vetpar.2003.11.015.
De Waal D.T. 1992. Equine piroplasmosis: a review. British Veterinary Journal, 148, 6–14. DOI: 10.1016/0007-1935(92)90061-5.
De Waal D.T., Van Heerden J., Potgieter F.T. 1987. An investigation into the clinical pathological changes and serological response in horses experimentally infected with Babesia equi and Babesia caballi. Onderstepoort Journal of Veterinary Research, 54, 561–568.
Friedhoff K.T., Soule C. 1996. An account on equine babesioses. OIE Revue Scientifique et Technique, 15, 1191–1201.
Holbrook A.A. 1969. Biology of equine piroplasmosis. Journal of the American Veterinary Medical Association, 155, 453–454.
Holman P.J., Hietala S.K., Kayashima L.R., Olson D., Waghela S.D., Wagner G.G. 1997. Case report: Field-acquired subclinical Babesia equi infection confirmed by in vitro culture. Journal of Clinical Microbiology, 35, 474–476.
Kouam M.K., Kantzoura V., Masuoka P.M., Gajadhar A.A., Theodoropoulos G. 2010. Genetic diversity of equine piroplasms in Greece with a note on speciation within Theileria genotypes (T. equi and T. equi like). Infection, Genetics and Evolution, 10, 963–968. DOI: 10.1016/j.meegid.2010.06.008.
Mehlhorn H., Schein E. 1998. Redescription of Babesia equi Laveran, 1901 as Theileria equi Mehlhorn, Schein 1998. Parasitology Research, 84, 467–475. DOI: 10.1007/s004360050431.
Oladosu L.A., Olufemi B.E. 1992. Haematology of experimental babesiosis and ehrlichiosis in steroid immunosuppressed horses. Journal of Veterinary Medicine Series B, 39, 345–352. DOI: 10.1111/j.1439-0450.1992.tb01179.x.
Pitel P.H., Scrive T., Léon A. 2010. Molecular detection of Theileria equi and Babesia caballi in the bone marrow of asymptomatic horses. Veterinary Parasitology, 170, 182–184. DOI: 10.1016/j.vetpar.2010.01.043.
Rampersad J., Cesa E., Campbell M.D., Samlal M., Ammons D. 2003. A field evaluation of PCR for the routine detection of Babesia equi in horses. Veterinary Parasitology, 114, 81–87. DOI: 10.1016/S0304-4017(03)00129-8.
Rhalem A., Sahibi H., Lasri S., Johnson W.C., Kappmeyer L.S., Hamidouch A., Knowles D.P., Goff W.L. 2001. Validation of a competitive enzyme-linked immunosorbent assay for diagnosing Babesia equi infections of Moroccan origin and its use in determining the seroprevalence of B. equi in Morocco. Journal of Veterinary Diagnostic Investigation, 13, 249–251. DOI: 10.1177/104063870101300311.
Salim B., Bakheit M.A., Kamau J., Nakamura I., Sugimoto C. 2010. Nucleotide sequence heterogeneity in the small subunit ribosomal RNA gene within Theileria equi from horses in Sudan. Parasitology Research, 106, 493–498. DOI: 10.1007/s00436-009-1691-7.
Schein E. 1988. Equine babesiosis. In: (Ed. M. Ristic) Babesiosis of Domestic Animals and Man. CRC Press, Boca Raton, 197–208.
Schetters T.P.M., Kleuskens J., Scholtes N., Gorenflot A. 1998. Parasite localization and dissemination in the Babesia-infected host. Annals of Tropical Medicine and Parasitology, 92, 513–519. DOI: 10.1080/00034989859483.
Seifi H.A., Mohri M., Sardari K.A. 2000. Mixed infection of Babesia equi and Babesia caballi in a racing colt: A report from Iran. Journal of Equine Veterinary Science, 20, 858–860. DOI:10.1016/S0737-0806(00)80117-3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ribeiro, I.B., Câmara, A.C.L., Bittencourt, M.V. et al. Detection of Theileria equi in spleen and blood of asymptomatic piroplasm carrier horses. Acta Parasit. 58, 218–222 (2013). https://doi.org/10.2478/s11686-013-0127-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11686-013-0127-9