Abstract
Taylor and Klein (Journal of Experimental Psychology: Human Perception and Performance 26:1639–1656, 2000) discovered two mutually exclusive “flavors” of inhibition of return (IOR): When the oculomotor system is “actively suppressed,” IOR affects input processes (the perception/attention flavor), whereas when the oculomotor system is “engaged,” IOR affects output processes (the motor flavor). Studies of brain activity with ignored cues have typically reported that IOR reduces an early sensory event-related potential (ERP) component (i.e., the P1 component) of the brain’s response to the target. Since eye movements were discouraged in these experiments, the P1 reduction might be a reflection of the perception/attention flavor of IOR. If, instead of ignoring the cue, participants made a prosaccade to the cue (and then returned to fixation) before responding to the target, the motor flavor of IOR should then be generated. We compared these two conditions while monitoring eye position and recording ERPs to the targets. If the P1 modulation is related to the perceptual/attentional flavor of IOR, we hypothesized that it might be absent when the motoric flavor of IOR was generated by a prosaccade to the cue. Our results demonstrated that target-related P1 reductions and behavioral IOR were similar, and significant, in both conditions. However, P1 modulations were significantly correlated with behavioral IOR only when the oculomotor system was actively suppressed, suggesting that P1 modulations may only affect behaviorally exhibited IOR when the attentional/perceptual flavor of IOR is recruited.
Similar content being viewed by others
To efficiently navigate the visual environment, humans have ostensibly adapted a mechanism that biases against returning to previously processed space (Klein & MacInnes, 1999; Posner, Rafal, Choate, & Vaughan, 1985). A great deal of laboratory research has demonstrated that keypress and saccadic eye movement response times (RTs) are slower to targets presented in previously processed rather than unprocessed space when the interval between the target and prior inputs exceeds approximately 300 ms (for a review, see Klein, 2000). These effects are often interpreted as reflecting a bias against previously processed inputs. Although multiple neural mechanisms may underlie this effect (e.g., Hunt & Kingstone, 2003; Kingstone & Pratt, 1999; Sumner, Nachev, Vora, Husain, & Kennard, 2004; Taylor & Klein, 2000; Zhang & Zhang, 2011), the term “inhibition of return” (IOR; Posner et al., 1985) is commonly used when referring to them.
Attentional/perceptual- versus motoric-flavored IOR
The cornerstone finding that is most commonly attributed to IOR (Posner et al., 1985) was first discovered by Posner and Cohen (1984) in a spatial-cueing paradigm in which the participant was instructed to ignore a spatially uninformative visual onset stimulus (the cue) and to respond to another onset (the target) that appeared sometime later. The imperative response stimulus (the target) appeared randomly at either the cued or uncued location. In this seminal study, observers depressed a single key to acknowledge the appearance of the target. Critically, Posner and Cohen also manipulated the time between the cue and target onsets, often referred to as the “cue–target onset asynchrony” (CTOA). Replicating previous findings (cf. Posner, 1980), RTs were faster to targets at the cued than at the uncued location if the CTOA was shorter than 200 ms. The surprising, and critical, finding was that when the CTOA exceeded 300 ms, RTs were slower to targets appearing at the cued, as compared to the uncued, location.
Using variations on this model task, Taylor and Klein (2000) parametrically manipulated the physical nature of the first and second signals (S1 and S2, respectively) in a dedicated effort to uncover the cause(s) and effect(s) of IOR (see Fig. 1). S1 and S2 comprised centrally presented arrows (pointing left or right) and peripherally presented luminance increases (occurring to the left or right of fixation). These signals were randomly intermixed in each block (see below); the response modes for S1 and S2 were manipulated between blocks. To generate the effect, S1 required (1) no response, (2) a saccadic response, or (3) a manual response. To measure the effect, S2 required either (1) a saccadic response or (2) a manual response. These manipulations yielded six unique response combinations of S1 and S2 (no response–manual, manual–manual, saccade–manual, no response–saccade, manual–saccade, and saccade–saccade) that were run, as noted above, in separate blocks. The CTOA was held constant at 1 s, and feedback was provided if a saccadic eye movement was made to a non-eye-movement signal.
Each observer was tested in each of the six conditions on separate days. The sequence of events was identical in each of these conditions: A trial would begin with the appearance of three rectangular landmarks, one at fixation and one apiece to the left and right of fixation. The first event was a peripheral box brightening (exogenous) or a central directional arrow signal (endogenous) whose location and direction, respectively, did not correlate with a future second signal (S2), occurring 1 s after the first (S1). S2, used to measure the effect of S1, was a randomly presented central arrow (endogenous) or a peripheral onset disc (exogenous). A cue back to fixation (brightening of the center box) invariably followed, 500 ms after the cue onset. The observer participated in each factorial combination of response types to S1 (no response, manual, or saccade) and S2 (manual or saccade), which, as noted, yielded six conditions. Note, principally, that it was not possible to measure the effect of S1 with a central arrow S2 when saccadic eye movements were not required (the two conditions highlighted by the bold rectangle), whereas this was possible in all conditions in which saccadic eye movements were required. The two hatched cells above are those being compared in the present experiment (no response to peripheral S1 with manual response to peripheral S2 vs. saccadic response to peripheral S1 with manual response to peripheral S2)
Considering all of the stimulus–response combinations described above, Taylor and Klein (2000) determined that IOR had two mutually exclusive effects. One effect of IOR, seen in conditions in which a saccadic response was required to S1, to S2, or to both, exhibited a primarily—if not entirely—motoric behavioral expression. Whether the cueing effect was measured by a peripheral onset or a central arrow S2, and whether the response to the target was executed by a saccade or a keypress, the magnitude of the IOR, considering all possible pairs of conditions, was no larger with peripheral than with central S2s [in fact, numerically, it was larger with central (22 ms) than with peripheral (17 ms) S2s]. It therefore appeared as if there was no effect of spatially overlapping signals on IOR, and it thus seemed as if IOR was affecting only the response (priority, or output/decision processes), and not the quality of the signal (salience, or input processes). Another effect of IOR, measured in conditions in which a saccadic eye movement response was strictly forbidden, exhibited a primarily—if not entirely—attentional/perceptual behavioral expression. In these cases, IOR was only observed with peripheral onset S2s. If there were a motoric component, one would—at bare minimum—expect IOR to occur when measured by responses to a central arrow S2.
According to Klein and Hilchey’s (2011) theoretical framework for IOR effects—which builds off of Taylor and Klein (2000)—when oculomotor responses are not permitted to task-relevant space, the reflexive oculomotor system is actively suppressed. In consequence, ancillary attentional/perceptual pathways are recruited to meet the task demands. By contrast, when oculomotor activation is necessary to meet the task demands, the effect of IOR is primarily motoric.
The validity of this oculomotor theory of IOR was recently evaluated by Chica, Taylor, Lupiáñez, and Klein (2010). Chica et al. administered a relatively standard spatial-cueing paradigm, wherein a peripheral onset cue preceded a to-be-discriminated target. In the key conditions, the imperative response signal was a manual discrimination response to a red- or green-colored circle, and Chica et al. manipulated, in separate blocks, whether or not a saccadic response was required to the peripheral onset cue. If, following Taylor and Klein (2000), the effect of IOR were on the quality of perceptual processing or the rate of information accrual when eye movements are not required, one would expect RTs to be slower at the cued as compared to the uncued location, while accuracy would either be reduced or equivalent at the cued as compared to the uncued location. If the effect of IOR was to bias against responding to previously cued locations and not to influence the accrual of perceptual information when saccadic eye movements were required, then responses would be slower to targets occurring at the cued location, while accuracy would be greater (a speed–accuracy trade-off would characterize the performance difference between cued and uncued targets). As such, a slower response to targets at the cued location would allow more perceptual processing to occur, which would, in effect, increase accuracy at the cued as compared to the uncued location. As would be predicted from Taylor and Klein’s theoretical framework, this study demonstrated that IOR expressed itself as a speed–accuracy trade-off when saccadic responses were made to S1 (cues), but not when the oculomotor system was actively suppressed. Inspired by these findings, a primary objective of the present study was to pit these attentional (no speed–accuracy trade-off observed) and oculomotor (speed–accuracy trade-off observed) cells against each other while recording electroencephalography (EEG) to reveal the extent to which attentional processes were modulating sensory inputs.
Neural signature of IOR
Event-related potential (ERP) investigations of orienting and attention have been particularly fruitful in examining the time course of attentional effects on different stages of processing (for a review, see Luck, Woodman, & Vogel, 2000). Research has demonstrated that sensory input processing, as revealed by the P1 ERP component (i.e., the first stimulus-induced positive peak in the EEG waveform), which arises from areas such as extrastriate cortex (e.g., Clark & Hillyard, 1996), can be modulated by attention (e.g., Mangun & Hillyard, 1988; Rugg, Milner, Lines, & Phalp, 1987). These studies have determined that the target-elicited early sensory P1 component is significantly enhanced when the target stimulus is attended, relative to when the same stimulus is not attended. In such paradigms, pretrial, task-induced attentional control settings lead to enhanced activity within the latency range of the P1 component, measured at electrodes over parieto-occipital cortex.
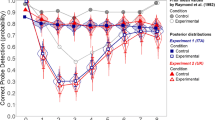
In other studies, researchers have recorded EEG while participants performed a spatial-cueing task with CTOAs in the range that normally leads to behavioral IOR. In all of these studies, participants have been instructed to maintain fixation throughout trials. To the extent that the observers obeyed this request, the investigations were likely measuring the perceptual/attentional aftereffect of spatial cueing. In addition to observing IOR behaviorally, many of these studies have also revealed significant P1 reductions (P1 cueing effects) for cued targets (McDonald, Ward, & Kiehl, 1999; Prime & Jolicœur, 2009a; Prime & Ward, 2004, 2006; Tian & Yao, 2008; van der Lubbe, Vogel, & Postma, 2005; Wascher & Tipper, 2004). However, as is illustrated in Fig. 2, not all experiments that have observed P1 cueing effects have also observed IOR (Doallo, Lorenzo-Lopez, Vizoso, Holguin, Amenedo, Bara & Cadaveira 2004; Hopfinger & Mangun, 1998), and not all experiments that have observed IOR have also observed P1 cueing effects (Hopfinger & Mangun, 2001; McDonald et al., 1999; Prime & Ward, 2006; van der Lubbe et al., 2005), so the relationship between IOR and P1 cueing effects has not yet been conclusively determined.
Comparison of IOR and P1 cueing effects (cued minus uncued), with r scores representing the correlation size and asterisks representing significance [for r = −.6, * p < .05 (two-tailed); for r = −.38, * p < .05 (one-tailed); see the text for more details]. (A) Xs represent the mean results of 19 published experiments from nine published aticles (Hopfinger & Mangun, 1998, 2001; McDonald et al., 1999; Prime & Jolicœur, 2009a, 2009b; Prime & Ward, 2004, 2006; Tian & Yao, 2008; van der Lubbe et al., 2005) in which a cue-ignored IOR paradigm was used. (B) Data points from the present experiment’s cue-ignored condition are represented with + s. (C) Data points from the present experiment’s cue-foveated condition are represented with diamonds
Although in these previous studies eye movements were verbally discouraged and there were attempts to remove all trials on which eye movements were made, this does not guarantee that trials with eye movements did not occur, and even more importantly, it does not guarantee that the reflexive machinery in the oculomotor system was actively suppressed. In the present investigation, we explicitly monitored eye position and provided online (trial-by-trial) feedback about incorrect eye movements or deviations from fixation, so we could unequivocally evaluate whether an eye movement was made, and could therefore ensure that oculomotor responses did not contribute to the data pattern.
To the extent that explicitly discouraging saccadic eye movements actively suppresses the oculomotor system, and thus leads to a perceptual/attentional form of IOR, we would expect to see target-elicited P1 reductions on the cued side as compared to the uncued side. Furthermore, we would expect a between-subjects relationship between IOR and these target-elicited P1 reductions at long CTOAs, akin to that which has been shown on a trial-by-trial basis in single-unit activity studies at short CTOAs (Dorris, Klein, Everling, & Munoz, 2002; Fecteau & Munoz, 2005). In spatial-cueing paradigms wherein monkeys exhibit behavioral IOR, these studies have uncovered a correlation between the neural activity of visuomotor neurons in the intermediate layer of the superior colliculus and RTs. Similarly, a significant correlation has also been obtained when looking at the mean experimental IOR scores and P1 cueing effects in the ERP literature (see Fig. 2A).
As we have described above, a number of previous studies have examined IOR behaviorally along with ERP analyses. Given these results, we performed a correlation analysis on the mean experiment-by-experiment IOR scores and cue-driven P1 modulation effects from all of the studies that have reported these numbers (19 published experiments from nine published articles: Hopfinger & Mangun, 1998, 2001; McDonald et al., 1999; Prime & Jolicœur, 2009a, 2009b; Prime & Ward, 2004, 2006; Tian & Yao, 2008; van der Lubbe et al., 2005). As is illustrated in Fig. 2A, the previous work has presented a clear pattern of larger (more negative) P1 cueing effects accompanying increased IOR scores (r = −.60, p < .05, two-tailed).
In addition to determining whether the data pattern that has been reported in the literature would be obtained in a condition in which the reflexive oculomotor machinery was truly “turned off,” we intended to use ERPs to provide converging evidence for the hypothesis that when the oculomotor system is in an activated state, the nature of the inhibitory aftereffect will be qualitatively different from that when the oculomotor system is actively suppressed. To reiterate, according to Taylor and Klein (2000) and Klein and Hilchey (2011), the oculomotor system is hypothesized to be in an activated state when prosaccadic responses are permitted/made to spatial inputs. Thus, by requiring observers to make a prosaccade to the first signal and a manual response to the second signal, we should, in theory, be measuring a primarily motoric effect. If this claim is valid, we might expect to see either (1) no target-elicited P1 reductions at the cued relative to the uncued location, or (2) if target-elicited P1 reductions were found at the cued location, these reductions should bear no relationship to IOR. In two separate blocks of trials, participants either ignored the cue (peripheral onset) or made saccadic eye movements to foveate it. EEG data were recorded and gaze position was monitored online, with trial-by-trial feedback provided to participants when their oculomotor behavior did not conform to the task requirements.
Method
Participants
A group of 24 students took part in this experiment in exchange for course extra credit. They were recruited from the participant pool at Dalhousie University. All of the participants reported normal or corrected-to-normal vision. Two participants were excluded from the analysis due to technical problems during EEG acquisition, and one other participant was excluded due to an excessive number of anticipatory responses. Consequently, the analyses presented here are based on the remaining 21 participants (13 females, 8 males, 20.6 years old on average).
Apparatus and stimuli
Presentation of the stimuli, timing, and behavioral data collection were controlled by a personal computer running Python scripts. Stimuli were presented on a 19 in. Asus LCD monitor, and responses were collected with a Microsoft keyboard. All stimuli were presented in white on a black background. Three boxes (4.5º × 4.5º of visual angle) were used as placeholders, a fixation cross (0.8º × 0.8º) was presented inside the central box, and the distance between the centers of adjacent boxes was 8.7º. Cues appeared as a thickening of one of the peripheral boxes, and the targets were bright disks with a diameter of 2.4º. Participants were tested in a dark, electromagnetically shielded room, with their heads resting on a chin rest that maintained the viewing distance at about 57 cm.
Gaze position was constantly monitored during the experiment using a desktop-mounted eyetracking system (EyeLink 1000), sampling at 250 Hz. EEG data were recorded continuously at 256 Hz with a BioSemi Active-Two amplifier system that used 64 Ag/AgCl electrodes mounted in an elastic cap according to the International 10–20 System. Electrodes were also placed at the outer canthi of the eyes, as well as above and below the left eye and on the mastoids. Two additional electrodes served as the recording reference and ground.
Design and procedure
The experimental procedure, as illustrated in Fig. 3, was similar to the design used by Chica et al. (2010). A self-paced drift correction was performed at the beginning of each trial, after which participants maintained fixation for 500 ms before a cue (nonpredictive of target location) appeared at one of the peripheral boxes for a duration of 300 ms. After a 900 ms interstimulus interval, the target appeared in one of the peripheral boxes. The target was presented for 3,000 ms or until the participant issued a localization response by pressing either the “z” or the “/” key for left or right targets, respectively. After an intertrial interval of 1,000–1,500 ms, another trial began.
Task procedure. The cue-ignored condition required maintenance of central fixation throughout the trials. In the cue-foveated condition, saccades were required to the cued location and back to central fixation. This figure is for illustrative purposes only; the stimuli are not drawn to scale (see the text for details)
The experiment consisted of two blocked conditions, the order of which was counterbalanced across participants. In one condition, participants made eye movements to the cued box and then back to the central fixation before target appearance (cue-foveated), while in the other condition participants maintained central fixation throughout the trials (cue-ignored). Each participant was tested for 200 trials per condition. Before beginning the experiment, participants were provided with enough practice trials to feel comfortable performing the task.
In the cue-ignored condition, a trial was aborted if the participant’s eyes deviated more than 3º from central fixation. In the cue-foveated condition, a trial was additionally aborted if the participant failed to make an eye movement to the cued box and then return to central fixation within 600 ms. An error message stating “Invalid eye movement. Press space to continue.” was presented whenever a trial was aborted.Footnote 1
EEG and behavioral analysis
EEG data were filtered with a high-pass filter of 0.1 Hz and a low-pass filter of 30 Hz. Bad electrodes were identified through visual inspection and interpolated using an automatic interpolation technique. The data were then re-referenced to the average of all electrodes and segmented into epochs beginning 100 ms before, and ending 400 ms after, target appearance. After performing a 100 ms baseline correction, trials with excessive artifacts (±75 μV) were excluded from the analysis. Trials with incorrect behavioral responses or any incorrect eye movements (on the basis of data from the EyeLink 1000 system) were also excluded from further analysis. The P1 ERP component was quantified by measuring each participant’s mean EEG amplitude at parieto-occipital electrodes (PO7/8) over a 20 ms window centered around the peak of the P1 component in the grand average waveforms for contralateral (130–150 ms) and ipsilateral (140–160 ms) electrodes.
Results
Behavioral performance
Trials with incorrect responses to targets were excluded from the analysis (1.06 % of the data). Careful examination of the overall RT distribution of correct responses led to the removal of another 3.84 % of trials on which the RTs were faster than 200 ms or slower than 600 ms (i.e., trials with RTs that were too fast or too slow to be considered representative). Trials rejected from the ERP analysis (e.g., those with excessive artifacts or faulty electrode activity) were also excluded from all behavioral analyses (11.14 % of the data). It should be noted that inclusion of trials with EEG artifacts in the behavioral analyses did not change the pattern of results. The remaining correct RTs were subjected to an ANOVA with the factors Condition (cue-ignored vs. cue-foveated) and Cueing (cued vs. uncued). This analysis revealed a main effect of cueing [F(1, 20) = 113.6, p < .001, η G 2 = .12 (generalized eta-squared; Bakeman, 2005)], in which observers were slower to respond to cued than to uncued targets (behavioral IOR; see Fig. 4). The main effect of condition did not approach significance [F(1, 20) < 1, n.s., η G 2 = .00], suggesting that there was no overall RT difference between the cue-ignored and cue-foveated conditions. As can be seen in Table 1, behavioral IOR effects were observed in both the cue-ignored (attentional/perceptual) and cue-foveated (motoric) conditions. However, the interaction between cueing and condition also reached significance [F(1, 20) = 21.6, p < .001, η G 2 = .02], suggesting that stronger IOR was observed when the cue was foveated (46 ms) [t(20) = 11.82, p < .001] than when it was ignored (21 ms) [t(20) = 4.85, p < .001].
Event-related potentials (ERPs)
The primary purpose of the present study was to explore how uninformative cues would modulate target-elicited P1s when the oculomotor system was theoretically in either an active or a suppressed state (Taylor & Klein, 2000) and to explore how any cue would modulate target-elicited P1 activation related to behavioral IOR effects. As can be seen in Fig. 5, cued targets elicited smaller P1 components than did uncued targets in both conditions. This cue-modulated P1 effect (reduced amplitudes for cued targets) was accompanied by a behavioral effect (increased RTs for cued targets—i.e., IOR; see Table 1).
For the statistical analyses, the P1 ERP component was quantified as the average activity over a 20 ms time window centered around the P1 peaks in the grand average waveforms of parieto-occipital electrodes (PO7/8). As is illustrated in the topographical activity maps presented in the Appendix, neural activity was maximal over ipsilateral parieto-occipital sites during the period of the ERP analysis. For easy comparison to behavioral IOR effects (RT differences between cued and uncued targets), we calculated the mean amplitude difference between cued and uncued target-elicited P1 ERP components (cued minus uncued), which we refer to as the “P1 cueing effect.” Table 1 summarizes the P1 cueing effects numerically.
ANOVAs with the factors Condition (cue-ignored vs. cue-foveated) and Cueing (cued vs. uncued) were performed on P1 components separately for ipsilateral and contralateral electrodes (PO7/PO8). For ipsilateral electrodes, a significant main effect of condition was observed [F(1, 20) = 14.15, p < .01, η G 2 = .20], with larger P1s in the cue-ignored (3.09 μV) than in the cue-foveated (1.26 μV) condition. This main effect was probably caused by an overall negative drift following the eye movements made in the cue-foveated condition. It should be noted that the negative drifts were similar for cued and uncued targets. A significant main effect of cueing was also observed [F(1, 20) = 16.98, p < .001, η G 2 = .10], with overall smaller P1s observed for cued (1.55 μV) than for uncued (2.80 μV) targets. The interaction between condition and cueing did not reach significance [F(1, 20) = 0.78, n.s., η G 2 = .00], suggesting that the P1 cueing effects were similar in magnitude across conditions. Planned comparisons revealed significant P1 reductions for cued targets in both the cue-ignored (1.05 μV) [t(20) = 3.24, p < .01] and cue-foveated (1.45 μV) [t(20) = 3.41, p < .01] conditions. For contralateral electrodes, only a main effect of condition was observed [F(1, 20) = 5.94, p < .05, η G 2 = .04]. This main effect also reflected the negative shift observed in the cue-foveated condition (see Fig. 5).
Relation between P1 modulations and the cueing effect
As was demonstrated in previous studies (e.g., McDonald et al., 1999; Prime & Jolicœur, 2009a; Prime & Ward, 2004, 2006; Tian & Yao, 2008; van der Lubbe et al., 2005; Wascher & Tipper, 2004) and in the present experiment, behavioral IOR effects are normally accompanied by a reduction of the early sensory P1 component. Although the precise localization of early sensory attentional modulation is still inconclusive, Fig. 6 demonstrates that the P1 cueing effects seen here were maximal at ipsilateral parieto-occipital sites, as had been observed previously (e.g., Chica & Lupiáñez, 2009). To provide a more rigorous test of the relationship between IOR (behavioral cueing effects) and P1 modulation effects, we calculated the participant-by-participant correlation between the IOR and P1 cueing effects for both the cue-foveated (motoric flavor) and cue-ignored (attentional/perceptual flavor) conditions. In the cue-foveated condition, IOR did not correlate with ipsilateral P1 cueing effects (r = .13, n.s.; see Fig. 2C). However, in the cue-ignored condition, a clear negative correlation was observed between the IOR and ipsilateral P1 cueing effects [r = −.38, p < .05 (one-tailed), p < .10 (two-tailed); see Fig. 2B]. As can be seen in Fig. 2B, the association observed in the cue-ignored condition (relative to uncued targets, larger reductions in P1 amplitude are associated with larger RT increases for cued targets) does not seem to be driven by outliers.
Discussion
Previous EEG studies have demonstrated that P1 component amplitudes are reduced on cued trials in spatial-cueing paradigms when IOR is expressed behaviorally (McDonald et al., 1999; Prime & Jolicœur, 2009a; Prime & Ward, 2004, 2006; Tian & Yao, 2008; van der Lubbe et al., 2005; Wascher & Tipper, 2004). The present study is the first ERP study of IOR that (1) has used eyetracking equipment to ensure that observers were conforming to the task demand and (2) has compared a variation of the spatial-cueing paradigm in which the oculomotor system has been hypothesized to be engaged (prosaccadic response to S1, keypress response to S2) with one in which the oculomotor system has been hypothesized to be actively suppressed. With regard to the activation state of the oculomotor system in the no-response-to-S1, manual-response-to-S2 condition, since participants made very few errors, we have little reason to believe that the state of the oculomotor system was anything other than actively suppressed. Following the theoretical framework proposed in the introduction, this allows for comparisons between P1 activation levels and spatial cueing when the oculomotor system is inferred to be “actively suppressed” (no response to S1, manual response to S2) or “engaged” (saccadic response to S1, manual response to S2).
Replicating previous findings, IOR was found to be accompanied by a P1 cueing effect. In addition, the present experiment produced several important observations. First, significant P1 cueing effects were observed whether the cues were ignored (suppressed oculomotor system) or foveated (active oculomotor system). Second, a significant correlation between the P1 reduction and IOR was only observed in the cue-ignored condition (where fixation was maintained throughout a trial). When saccades were made to the cue, there was no correlation between P1 cueing effects and IOR scores. Third, stronger IOR was observed when the oculomotor system was actively involved, in the cue-foveated condition. The theoretical and empirical implications of these findings are considered below.
Why did foveated S1s lead to stronger IOR?
Since the two conditions of this experiment were blocked, differences in IOR scores across conditions are likely to be explained by different attentional control settings induced by the different task demands in the two conditions (cf. Hilchey, Klein, & Ivanoff, 2012; Wang & Klein, 2012). In the cue-ignored condition, participants have little reason to attend the periphery at the time of cue presentation, since no response is required to the cue and the cues are uninformative with regard to target location. However, in the cue-foveated condition, saccadic responses are required to the cues, so the peripheral cue locations must be attended at the time of cue presentation. Thus, the cue-ignored condition should result in a much tighter attentional control setting focused on central fixation, whereas the cue-foveated condition should result in a more diffuse attentional control setting that encompasses the peripheral cue locations. This could result in a weaker-intensity attentional beam at peripheral cue locations in the cue-ignored condition, relative to the cue-foveated condition. Since previous work has demonstrated that IOR can be reduced or eliminated for cues presented outside of a spatial attentional control setting, the larger IOR score seen in the cue-foveated (46 ms) than in the cue-ignored (21 ms) condition is not surprising.Footnote 2
However, this finding is seemingly in contradiction to that of Taylor and Klein (2000), who found mathematically larger IOR effects with manual responses following ignored (21 ms) rather than foveated (14 ms) cues (a difference that was not subjected to a statistical test by Taylor & Klein, 2000). How has this occurred? There are two obvious methodological differences between Taylor and Klein’s study and the present investigation: (1) Taylor and Klein adopted the convention of delivering a cue back to fixation, which, in theory, helped to ensure that any attention that might have been drawn to the first, spatially irrelevant peripheral onset signal was returned to fixation (a neutral baseline; see Klein, 2004), and (2) in Taylor and Klein’s study, centrally presented arrow signals were randomly intermixed with peripheral onset signals, whereas we only presented peripheral onset signals. Since both of these experimental manipulations are known to have strong effects on IOR scores (Hilchey et al., 2012; Prime & Jolicœur, 2009a; Wang & Klein, 2012; Wang, Satel, & Klein, 2012), it is likely that the differences in the behavioral results arise from these differences in experimental design.
As was discussed by Wang et al. (2012), the presence of a visual cue-back could contaminate the IOR scores in conditions in which the cue is foveated. Since the central fixation location is in the same retinotopic representation as the uncued target location at the time of cue-back onset, an inhibitory cueing effect could be operating on the uncued target location. Thus, when comparing cued to uncued RTs, we may actually be comparing locations that have each been subjected to inhibitory mechanisms (cf. Satel, Wang, Trappenberg, & Klein, 2011; Wang et al., 2012; Wang, Satel, Trappenberg, & Klein, 2011). This could explain the difference across studies in the cue-foveated condition IOR scores, with much less IOR being observed by Taylor and Klein (2000; 14 ms) than here (46 ms) because of a competing inhibitory mechanism operating at the uncued location.
In the present investigation, it is worth reiterating that there were never task-relevant events at fixations (i.e., there were neither central arrows nor cue-backs to fixation). By contrast, as was mentioned before, imperative signals occurred at fixation in both conditions (saccadic response to S1, manual response to S2) of Taylor and Klein (2000). As we stated above, we believe that it is possible that the absence of relevant inputs occurring at fixation would allow for greater processing of the peripheral S1 events in our experiment as compared to Taylor and Klein’s, because no task demand (i.e., relevant fixation events or the requirement to remain fixated) strongly encouraged increased processing at the fixation stimulus (at the cost of peripheral processing; see Hilchey et al., 2012; Wang & Klein, 2012).
IOR and P1 modulation as a function of the state of the oculomotor system
Previous findings from a wide range of studies have indirectly supported the relationship between P1 cueing effects and the behavioral expression of IOR. On the one hand, that there were many differences between these studies suggests caution when interpreting these results as a whole (see Fig. 2A). On the other hand, this methodological eclecticism, together with so many observations, suggests that the significant correlation observed here is probably closely related to what the studies have in common: cued versus uncued targets with CTOAs long enough for IOR to be present. The correlation between IOR and P1 cueing effects for these previous experiments, none of which required eye movements, is significant (r = −.60, p < .05). Thus, at least in cases in which the oculomotor system is not required to be actively engaged to meet task requirements, it seems there is a legitimate association between P1 cueing effects and IOR.
To further investigate the relationship between IOR and P1 modulations, we looked at the participant-by-participant correlations between these measurements in the present experiment. Because Taylor and Klein (2000) claimed that the effect of IOR is primarily attentional/perceptual when the oculomotor system is “actively suppressed,” we expected a correlation between IOR and P1 modulation in the no-response-to-S1, manual-response-to-S2 condition. By contrast, because Taylor and Klein claimed that the effect of IOR is primarily (if not entirely) motoric when the oculomotor system is “engaged,” we expected that there would be no P1 modulations in the saccadic-response-to-S1, manual-response-to-S2 condition.
This inference was recently supported by an investigation highlighted in the introduction (Chica et al., 2010), on which the present investigation was largely based. Chica et al. demonstrated that in the aftermath of a saccadic eye movement to a region in the visual field, and a subsequent return eye movement to fixation, observers were slower but more accurate to identify a target occurring at previously foveated as compared to unfoveated regions. By contrast, when observers withheld oculomotor responses to spatially irrelevant peripheral onset cues and subsequently identified a target at the cued or uncued region, RTs were slower for the cued region, and response accuracy was either equivalent between the cued and uncued regions, or perhaps slightly worse at the cued region.
Taken together, the foregoing results suggest that in the presence of saccadic eye movements or, possibly, when eye movements are permitted (whether or not they are actually executed), the effect of IOR is akin to the “criterion shift” account (Klein & Taylor, 1994) that was expounded on by Ivanoff, Klein, and Lupiáñez (2002). In broad strokes, this theory posits that IOR affects decision making such that increased evidence is required before response execution (i.e., there is an increased response criterion for the cued relative to uncued location), while the accrual of information at cued and uncued locations remains equivalent. In stark contrast, the effect of IOR when eye movements are strictly forbidden (the oculomotor system is “inactive,” if not actively inhibited) is in better agreement with Posner and Cohen’s (1984) “inhibited attention” hypothesis. As conceptualized by Ivanoff et al., this theory posits that there are slower RTs to targets at the cued than at the uncued location because of a delay in the activation of the task-relevant stimulus–response code (cf. Hilchey, Ivanoff, Taylor, & Klein, 2011); this effect could arise simply because the quality of visual information processing at the cued location is degraded to some extent (i.e., a perceptual/attentional account).
Converging on the claim that the effect of IOR is primarily attentional/perceptual when the oculomotor system is “actively suppressed,” P1 cueing effects were correlated with IOR in the no-response-to-S1, manual-response-to-S2 condition. On the other hand, there was no apparent relationship between IOR and P1 modulations when the oculomotor system was “engaged.” This is a striking result.Footnote 3 The most immediate implication is that whereas IOR is commonly associated with P1 reductions in non-eye-movement spatial-cueing studies, this pattern—whether or not it is indexing the existence of an attentional effect of IOR in the brain—only expresses itself behaviorally when the oculomotor system is “actively suppressed.” Thus, following an act of overt orienting toward a peripheral stimulus (and return to the original fixation), there may be a reduction in input pathways, but this effect does not appear to significantly influence manual RTs. Future work is encouraged to further investigate how this comes about.
Conclusion
In this experiment, we examined IOR when generated by ignored or foveated cues, while simultaneously recording EEG and eye position and providing feedback to participants about any incorrect oculomotor behavior. Our motivation was twofold: (1) We wanted to ensure that previous EEG work on IOR was not compromised by the presence of unintended saccadic eye movements to cues or targets, and (2) we wanted to evaluate the claim from Taylor and Klein (2000) that the activation state of the oculomotor system would determine the effect of IOR (i.e., motoric or attentional/perceptual). When the oculomotor system was “actively suppressed,” we observed IOR, P1 cueing effects, and a statistically significant brain–behavior relationship between these dependent variables. When the oculomotor system was “engaged,” we observed statistically equivalent P1 cueing effects—relative to those obtained when the oculomotor system was “actively suppressed”—behavioral IOR that was statistically greater than when the oculomotor system was “actively suppressed,” and no relationship between P1 cueing effects and IOR. On balance, our data are consistent with the idea that P1 cueing effects in IOR paradigms result from the successive presentation of peripheral stimuli and that this P1 cueing effect is closely associated with RT when the oculomotor system is suppressed. By contrast, no apparent association exists between P1 cueing effects and IOR when the oculomotor system is activated due to requiring saccades to cues.
Notes
Although every aborted trial was put back into the queue to be rerun later and the remaining “upcoming” trials were randomized, the length of each block was still limited to 200 trials, to ensure that sessions would end in a timely fashion.
Although the correlation for our cue-ignored condition was only significant with a one-tailed test, when compared against each other (for details on this analytical technique, see Steiger, 1980), the correlation seen in previous work (r = −.60) was not significantly different from that obtained in our cue-ignored condition (r = −.38). Furthermore, the correlation obtained in our cue-foveated condition (r = .13) was significantly different from that in the cue-ignored condition (Z = −3.04). This provides converging evidence that two different mechanisms likely operate in the two conditions.
References
Bakeman, R. (2005). Recommended effect size statistics for repeated measures designs. Behavior Research Methods, 37, 379–384. doi:10.3758/BF03192707
Chica, A. B., & Lupiáñez, J. (2009). Effects of endogenous and exogenous attention on visual processing: An inhibition of return study. Brain Research, 1278, 75–85.
Chica, A. B., Taylor, T. L., Lupiáñez, J., & Klein, R. M. (2010). Two mechanisms underlying inhibition of return. Experimental Brain Research, 201, 25–35. doi:10.1007/s00221-009-2004-1
Clark, V. P., & Hillyard, S. A. (1996). Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. Journal of Cognitive Neuroscience, 8, 387–402. doi:10.1162/jocn.1996.8.5.387
Doallo, S., Lorenzo-Lopez, L., Vizoso, C., Holguin, S. R., Amenedo, E., Bara, S., & Cadaveira, F. (2004). The time course of the effects of central and peripheral cues on visual processing: An event-related potentials study. Clinical Neurophysiology, 115, 199–210.
Dorris, M. C., Klein, R. M., Everling, S., & Munoz, D. P. (2002). Contribution of the primate superior colliculus to inhibition of return. Journal of Cognitive Neuroscience, 14, 1256–1263. doi:10.1162/089892902760807249
Fecteau, J. H., & Munoz, D. P. (2005). Correlates of capture of attention and inhibition of return across stages of visual processing. Journal of Cognitive Neuroscience, 17, 1714–1727. doi:10.1162/089892905774589235
Hilchey, M. D., Ivanoff, J., Taylor, T. L., & Klein, R. M. (2011). Visualizing the temporal dynamics of spatial information processing responsible for the Simon effect and its amplification by inhibition of return. Acta Psychologica, 136, 235–244. doi:10.1016/j.actpsy.2010.09.003
Hilchey, M. D., Klein, R. M., & Ivanoff, J. (2012). Perceptual and motor inhibition of return: Components or flavors? Attention, Perception, & Psychophysics, 74, 1416–1429. doi:10.3758/s13414-012-0332-x
Hopfinger, J. B., & Mangun, G. R. (1998). Reflexive attention modulates processing of visual stimuli in human extrastriate cortex. Psychological Science, 9, 441–447.
Hopfinger, J. B., & Mangun, G. R. (2001). Tracking the influence of reflexive attention on sensory and cognitive processing. Cognitive, Affective, & Behavioral Neuroscience, 1, 56–65. doi:10.3758/CABN.1.1.56
Hunt, A. R., & Kingstone, A. (2003). Inhibition of return: Dissociating attentional and oculomotor components. Journal of Experimental Psychology. Human Perception and Performance, 29, 1068–1074. doi:10.1037/0096-1523.29.5.1068
Ivanoff, J., Klein, R. M., & Lupiáñez, J. (2002). Inhibition of return interacts with the Simon effect: An omnibus analysis and its implications. Perception & Psychophysics, 64, 318–327. doi:10.3758/BF03195794
Kingstone, A., & Pratt, J. (1999). Inhibition of return is composed of attentional and oculomotor processes. Perception & Psychophysics, 61, 1046–1054. doi:10.3758/BF03207612
Klein, R. M. (2000). Inhibition of return. Trends in Cognitive Sciences, 4, 138–147. doi:10.1016/S1364-6613(00)01452-2
Klein, R. (2004). Orienting and inhibition of return. In M. S. Gazzaniga (Ed.), The cognitive neurosciences (3rd ed., pp. 545–559). Cambridge: MIT Press.
Klein, R. M., & Hilchey, M. D. (2011). Oculomotor inhibition of return. In S. Liversedge, I. D. Gilchrist, & S. Everling (Eds.), The Oxford handbook of eye movements (pp. 471–492). Oxford: Oxford University Press.
Klein, R. M., & MacInnes, W. J. (1999). Inhibition of return is a foraging facilitator in visual search. Psychological Science, 10, 346–352. doi:10.1111/1467-9280.00166
Klein, R. M., & Taylor, T. L. (1994). Categories of cognitive inhibition, with reference to attention. In D. Dagenbach & T. H. Carr (Eds.), Inhibitory processes in attention, memory, and language (pp. 113–150). San Diego: Academic Press.
Luck, S. J., Woodman, G. F., & Vogel, E. K. (2000). Event-related potential studies of attention. Trends in Cognitive Sciences, 4, 432–440. doi:10.1016/S1364-6613(00)01545-X
Mangun, G. R., & Hillyard, S. A. (1988). Spatial gradients of visual attention: Behavioral and electrophysiological evidence. Electroencephalography and Clinical Neurophysiology, 70, 417–428.
McDonald, J. J., Ward, L. M., & Kiehl, K. A. (1999). An event-related brain potential study of inhibition of return. Perception & Psychophysics, 61, 1411–1423.
Posner, M. I. (1980). Orienting of attention. Quarterly Journal of Experimental Psychology, 32, 3–25. doi:10.1080/00335558008248231
Posner, M. I., & Cohen, Y. (1984). Components of visual orienting. In H. Bouma & D. G. Bouwhuis (Eds.), Attention and performance X: Control of language processes (pp. 531–556). Hillsdale: Erlbaum.
Posner, M. I., Rafal, R. D., Choate, L. S., & Vaughan, J. (1985). Inhibition of return: Neural basis and function. Cognitive Neuropsychology, 2, 211–228. doi:10.1080/02643298508252866
Prime, D., & Jolicœur, P. (2009a). On the relationship between occipital cortex activity and inhibition of return. Psychophysiology, 46, 1278–1287.
Prime, D., & Jolicœur, P. (2009b). Response-selection conflict contributes to inhibition of return. Journal of Cognitive Neuroscience, 21, 991–999.
Prime, D. J., & Ward, L. M. (2004). Inhibition of return from stimulus to response. Psychological Science, 15, 272–276. doi:10.1111/j.0956-7976.2004.00665.x
Prime, D. J., & Ward, L. M. (2006). Cortical expressions of inhibition of return. Brain Research, 1072, 161–174.
Rugg, M. D., Milner, A. D., Lines, C. R., & Phalp, R. (1987). Modulation of visual event-related potentials by spatial and non-spatial visual selective attention. Neuropsychologia, 25, 85–96. doi:10.1016/0028-3932(87)90045-5
Satel, J., Wang, Z., Trappenberg, T. P., & Klein, R. M. (2011). Modeling inhibition of return as short-term depression of early sensory input to the superior colliculus. Vision Research, 51, 987–996.
Steiger, J. H. (1980). Tests for comparing elements of a correlation matrix. Psychological Bulletin, 87, 245–251. doi:10.1037/0033-2909.87.2.245
Sumner, P., Nachev, P., Vora, N., Husain, M., & Kennard, C. (2004). Distinct cortical and collicular mechanisms of inhibition of return revealed with S cone stimuli. Current Biology, 14, 2259–2263. doi:10.1016/j.cub.2004.12.021
Taylor, T. L., & Klein, R. M. (2000). Visual and motor effects in inhibition of return. Journal of Experimental Psychology. Human Perception and Performance, 26, 1639–1656. doi:10.1037/0096-1523.26.5.1639
Tian, Y., & Yao, D. (2008). A study on the neural mechanism of inhibition of return by the event-related potential in the Go/Nogo task. Biological Psychology, 79, 171–178.
van der Lubbe, R. H., Vogel, R. O., & Postma, A. (2005). Different effects of exogenous cues in a visual detection and discrimination task: Delayed attention withdrawal and/or speeded motor inhibition? Journal of Cognitive Neuroscience, 17, 1829–1840.
Wang, Z., & Klein, R. M. (2012). Focal spatial attention can eliminate inhibition of return. Psychonomic Bulletin & Review, 19, 462–469. doi:10.3758/s13423-012-0226-x
Wang, Z., Satel, J., & Klein, R. M. (2012). Sensory and motor mechanisms of oculomotor inhibition of return. Experimental Brain Research, 218, 441–453.
Wang, Z., Satel, J., Trappenberg, T., & Klein, R. M. (2011). Aftereffects of saccades explored in a dynamic neural field model of the superior colliculus. Journal of Eye Movement Research, 4, 1–16.
Wascher, E., & Tipper, S. P. (2004). Revealing effects of noninformative spatial cues: An EEG study of inhibition of return. Psychophysiology, 41, 716–728.
Zhang, Y., & Zhang, M. (2011). Spatial working memory load impairs manual but not saccadic inhibition of return. Vision Research, 51, 147–153. doi:10.1016/j.visres.2010.10.022
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Topographical activity plots averaged over the time period used for the ipsilateral P1 analyses (140–160 ms post-cue). Top row: Mean of cued and uncued activity for left and right targets in both conditions. Bottom row: Difference between cued and uncued activity for left and right targets in both conditions
Rights and permissions
About this article
Cite this article
Satel, J., Hilchey, M.D., Wang, Z. et al. The effects of ignored versus foveated cues upon inhibition of return: An event-related potential study. Atten Percept Psychophys 75, 29–40 (2013). https://doi.org/10.3758/s13414-012-0381-1
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13414-012-0381-1