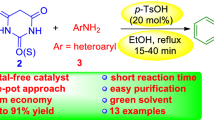
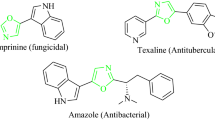
Abstract
The reaction of 5-hydroxyindoles with ammonia, alkylamines, or dialkylamines in the presence of sulfites leads to the corresponding 5-aminoindoles. Partial or complete elimination of the substituent is observed in the case of indoles that have an electron-acceptor substituent (COOC2H5, COCH3).
Similar content being viewed by others
Literature cited
R. Adams (ed.), Organic Reactions, Wiley (1942).
V. G. Pesin, A. M. Khaletskii, and I. A. Lotsmanenko, Zh. Obshch. Khim., 33, 1746 (1963).
V. G. Pesin and I. A. Belen'kaya, Khim. Geterotsikl. Soedin., No. 4, 666 (1967).
H. Gilman and J. Swiss, J. Am. Chem. Soc., 66, 1884 (1944).
J. Thesing, G. Sembler, and G. Mohr, Chem. Ber., 95, 2205 (1962).
J. Powers, Tetrahedron Lett., No. 11, 655 (1965).
R. Adams and W. Samuels, J. Am. Chem. Soc., 77, 5375 (1955).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 6, pp. 786–789, June, 1979.
Rights and permissions
About this article
Cite this article
Kost, A.N., Terenin, V.I. & Yudin, L.G. Synthesis of aminoindoles by the Bucherer reaction. Chem Heterocycl Compd 15, 639–642 (1979). https://doi.org/10.1007/BF00539499
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00539499