Summary
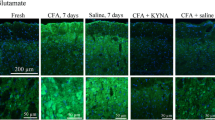
This study measured the release of glutamate (Glu) and aspartate (Asp) amino acid transmitters in the ventrocaudal compartment of the rat periaqueductal gray (PAG) following exposure to unilateral peripheral inflammation. The release of endogenous Glu and Asp from the rat ventrocaudal PAG was monitored with the microdialysis technique in unanesthetized, unrestrained rats. There was significant increase (1,300%) in the basal concentrations of Glu release in the 7 days Complete Freund's Adjuvant (CFA) treated group compared to 24h mineral oil control group. Amino acid release was induced by infusing veratridine (75μM, a sodium channel activator) directly through the 1 mm long dialysis probe. Perfusion of veratridine into the ventrocaudal PAG resulted in significant elevation of Glu and Asp amino acids. In the 24h and 7 days CFA treated rats, veratridine-evoked release of Glu was significantly decreased in the lateral ventrocaudal PAG compared to control rats injected with mineral oil (CFA vehicle). The peak minus baseline concentrations of Glu in 24h and 7 days CFA treated groups decreased 55.7% and 43.9%, respectively. In contrast, The basal and the peak minus baseline concentrations of Asp showed no significant change between control group and 24h and 7 days CFA treated animals. The results provide direct evidence that Glu excitatory amino acid may be involved in nociception/nociception modulation pathway in the ventrocaudal PAG.
Similar content being viewed by others
References
Aimone LD, Gebhart GF (1986) Stimulation-produced spinal inhibition from the midbrain in the rate is mediated by an excitatory amino acid neurotransmitter in the medial medulla. J Neurosci 7: 1803–1813
Albin RL, Makowiec RL, Hollingsworth Z, Dure LS, Penney JB, Young AB (1990) Excitatory amino acid receptors in the periaqueductal gray of the rat. Neurosci Lett 118: 112–115
Basbaum AI, Fields HL (1984) The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: further studies on the anatomy of pain modulation. J Comp Neurol 187: 513–532
Behbehani MM, Fields HL (1979) Evidence that an excitatory connection between periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res 170: 85–93
Beitz AJ (1989) Possible Origin of glutamatergic projections to the midbrain periaqueductal gray and deep layer of the superior colliculus of the rat. Brain Res Bull 23: 25–35
Beitz AJ (1990) Relationship of glutamate and aspartate to the periaqueductal gray raphe magnus projection: analysis using immuno cytochemistry and microdialysis. J Histochem Cytochem 38: 1755–1765
Beitz AJ, Williams FG (1991) Localization of putative amino acid transmitters in the PAG and their relationship to the PAG-raphe magnus pathway. In: Depaulis A, Bandler R (eds) The midbrain periaqueductal gray matter. Plenum, New York, pp 305–327
Bennet GJ, Mayer DJ (1979) Inhibition of spinal cord interneurons by narcotic injections. Brain Res 172: 243–257
Benveniste H, Huttemeier PC (1990) Microdialysis: theory and application. Prog Neurobiol 35: 195–215
Butler SH, Godefroy F, Besson JM, Wiel-Fugazza JA (1992) Limited arthritic model for chronic pain studies in the rat. Pain 48: 73–81
Clements JR, Madl JE, Johnson RL, Larson AA, Beitz AJ (1987) Localization of glutamate, glutaminase, aspartate and aspartate aminotransferase in the rat midbrain periaqueductal gray. Exp Brain Res 67: 594–602
Cotman CW, Monaghan DT, Ottersen OP, Storm-Mathisen J (1987) Anatomical excitatory amino acid receptors and their pathways. Trends Neurosci 10: 273–280
Di Chiara G (1990) In vivo brain dialysis of neurotransmitters. Trends Phar Sci 11: 116–121
Evans RH (1989) Pharmacology of segmental transmission in the spinal cord. Prog Neurobiol 33: 225–279
Fang FG, Haws CM, Drasner K, Williamson A, Fields HL (1989) Opioid peptides (DAGO-enkephalin, dynorphin A(1–13), BAM 22P) microinjected into the rat brainstem. Comparison of their antinociceptive effect and their effect on neuronal firing in the rat ventromedial medulla. Brain Res 501: 116–128
Fields HL, Anderson SD (1978) Evidence that raphe-spinal neurons mediate opiate and midbrain stimulation-produced analgesia. Pain 5: 333–349
Fields HL, Hienricher MM, Mason P (1991) Neurotransmitters in nociceptive modulatory circuits. Anal Rev Neurosci 14: 219–245
Gold MS, Morgan MM, Liebeskind JC (1990) Antinociceptive and behavioral effects of low dose kainic acid injections into the PAG of the rat. Pain [Suppl] 5: 441
Goodchild AK, Dampney RAL, Bandler RA (1982) A method for evoking physiological responses by stimulation of cell bodies, but not axons of passage, within localized regions of central nervous system. J Neurosci Meth 6: 351–363
Hary SGP (1985) Analgesia elicited by prefrontal stimulation. Brain Res 339: 281–284
Holstege G, Kuypers HGJM (1982) The anatomy of brainstem pathways to the spinal cord in cat. A labeled amino acid tracing study. Prog Brain Res 57: 45–75
Iadarola MJ, Brady LS, Draisci G, Dubner R (1988) Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: Stimulus specificity, behavioral parameters and opioid receptor binding. Pain 35: 313–326
Jacquet YF (1988) The NMDA receptor: central role in pain inhibition in the rat periaqueductal gray. Eur J Pharmacol 154: 271–276
Jensen TS, Yaksh TL (1992) The antinociceptive activity of excitatory amino acids in the rat brainstem: an anatomical and pharmacological analysis. Brain Res 569: 255–267
Keay KA, Bandler R (1993) Deep and superficial noxious stimulation increases Fos-like immunoreactivity in different regions of the midbrain periaqueductal gray of the rat. Neurosci Lett 154: 23–26
Lindorth O, Mopper K (1974) High performance liquid chromatographic determination in picomole amounts of amino acids by precolumn fluorescence derivatization with 0-phthaldialdehyde. Anal Chem 51: 1667–1674
Mantyh PW (1983) Connections of midbrain periaqueductal gray in the monkey. I. Ascending efferent projections. J Neurophysiol 49: 567–581
Ottersen OP, Storm-Mathisen J (1984) Glutamate-and GABA-containing neurotensin in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J Comp Neurol 229: 374–392
Patneau DK, Mayer ML (1990) Structure-activity relationships for amino acid transmitter candidates acting at N-methey-D-aspartate and Quisqualate receptors. J Neurosci 10: 2385–2399
Paxinos G, Watson C (1986) The rat brain in stereotoxic coordinates, 2nd edn. Academic Press, Sidney
Renno WM, Mullett MA, Beitz AJ (1992) Systemic morphine reduces GABA release in the lateral but not the medial portion of the midbrain periaqueductal gray of the rat. Brain Res 594: 221–232
Reynolds DV (1969) Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science 164: 444–445
Rizvi TA, Ennis M, Behbahani M, Shipley MT (1991) Connechous between the central nucleus of the amygdala and midbrain periaqueductal gray: topography and reciprocity. J Comp Neurol 303: 121–131
Skilling SR, Mullett M, Beitz AJ, Larson AA (1992) Fluorogold administration via microdialysis labels neurons terminating with the dialysis region. J Neurosci Methods 85–90
Stein C, Millan JJ, Herz A (1988) Unilateral inflammation of the hindpaw in rats as a model of prolonged noxious stimulation: alterations in behavior and nociceptive thresholds. Pharmacol Biochem Behav 31: 445–451
Ungerstedt U (1984) Measurement of neurotransmitter release by intracranial dialysis. In: Marsden CA (ed) Measurement of neurotransmitter release in vivo. Wiley, New York, pp 81–105
Van Praag H, Frenk H (1990) The role of glutamate in opiate descending inhibition of nociceptive spinal reflexes. Brain Res 524: 101–105
Wiklund L, Behzadi G, Kalen P, Headley PM, Nicolopoulos LS, Parsons CG, West DC (1988) Autoradiographic and electrophysiological evidence for excitatory amino acid transmission in the periaqueductal gray projection to nucleus raphe magnus in the rat. Neurosci Lett 93: 158–163
Williams FG, Beitz AJ (1993) Chronic pain increases brainstem proneurotensin/neuromedin-N mRNA expression: a hybridization-histochemical and immunohisto-chemical study using three different rat models for chronic nociception. Brain Res 611: 87–102
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Renno, W.M. Microdialysis of excitatory amino acids in the periaqueductal gray of the rat after unilateral peripheral inflammation. Amino Acids 14, 319–331 (1998). https://doi.org/10.1007/BF01318851
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01318851