Abstract
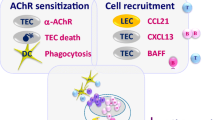
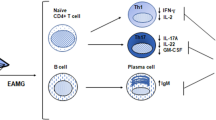
Myasthenia gravis (MG) is an autoimmune disease caused by an immunological response against the acetylcholine receptor (AChR) at the neuromuscular junction. Anti-AChR antibodies induce degradation of the receptor, activation of complement cascade and destruction of the post-synaptic membrane, resulting in a functional reduction of AChR availability. The pathophysiological role of autoantibodies (auto-Abs) and T helper lymphocytes has been studied in the experimental autoimmune MG (EAMG) models. EAMG models have been employed to investigate the factors involved in the development of MG and to suggest new therapies aimed to preventing or modulating the ongoing disease. EAMG can be induced in susceptible mouse and rat strains, which develop clinical symptoms such as muscular weakness and fatigability, mimicking the human disease. Two major types of EAMG can be induced, passive and active EAMG. Passive transfer MG models, involving the injection of auto-Abs, are helpful for studying the role of complement molecules and their regulatory proteins, which can prevent neuromuscular junction degradation. Active models, induced by immunization, are employed for the analysis of antigen-specific immune responses and their modulation in order to improve disease progression. In this review, we will concentrate on the main pathogenic mechanisms of MG, focusing on recent findings on EAMG experimental models.

Similar content being viewed by others
Abbreviations
- aa:
-
Amino acids
- AChR:
-
Acetylcholine receptor
- TAChR:
-
Torpedo californica AChR
- auto-Abs:
-
Autoantibodies
- BMSC:
-
Bone marrow stromal cells
- DC:
-
Dendritic cells
- MG:
-
Myasthenia gravis
- MIR:
-
Main immunogenic region
- EAMG:
-
Experimental autoimmune MG
- CFA:
-
Complete Freund’s adjuvant
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- IgG:
-
Immunoglobulin G type
- MAC:
-
Membrane attack complex
- NMJ:
-
Neuromuscular junction
- PBL:
-
Peripheral blood lymphocytes
- PIX:
-
Pixantrone (BBR2778)
- SCID:
-
Severe combined immunodeficiency
- TGF-β1:
-
Transforming growth factor 1-beta
- Treg:
-
Regulatory T-cell
References
Abramsky O, Tarrab-Hazdai R, Aharonov A et al (1976) Immunosuppression of experimental autoimmune myasthenia gravis by hydrocortisone and azathioprine. J Immunol 117:225–228
Antozzi C, Gemma M, Regi B et al (1991) A short plasma exchange protocol is effective in severe myasthenia gravis. J Neurol 238:103–107
Arce S, Cassese G, Hauser A et al (2002) The role of long-lived plasma cells in autoimmunity. Immunobiology 206:558–562
Aricha R, Feferman T, Fuchs S et al (2008) Ex vivo generated regulatory T cells modulate experimental autoimmune myasthenia gravis. J Immunol 180:2132–2139
Aricha R, Feferman T, Scott HS et al (2011) The susceptibility of Aire(−/−) mice to experimental myasthenia gravis involves alterations in regulatory T cells. J Autoimmun 36:16–24
Baggi F, Andreetta F, Caspani E et al (1999) Oral administration of an immunodominant T-cell epitope downregulates Th1/Th2 cytokines and prevents experimental myasthenia gravis. J Clin Invest 104:1287–1295
Baggi F, Annoni A, Ubiali F et al (2004) Breakdown of tolerance to a self-peptide of acetylcholine receptor alpha-subunit induces experimental myasthenia gravis in rats. J Immunol 172:2697–2703
Balandina A, Lecart S, Dartevelle P et al (2005) Functional defect of regulatory CD4(+)CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood 105:735–741
Balasa B, Deng C, Lee J et al (1997) Interferon gamma (IFN-gamma) is necessary for the genesis of acetylcholine receptor-induced clinical experimental autoimmune myasthenia gravis in mice. J Exp Med 186:385–391
Balasa B, Deng C, Lee J et al (1998) The Th2 cytokine IL-4 is not required for the progression of antibody-dependent autoimmune myasthenia gravis. J Immunol 161:2856–2862
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Barchan D, Souroujon MC, Im SH et al (1999) Antigen-specific modulation of experimental myasthenia gravis: nasal tolerization with recombinant fragments of the human acetylcholine receptor alpha-subunit. Proc Natl Acad Sci USA 96:8086–8091
Bellone M, Ostlie N, Lei SJ et al (1991) The I-Abm12 mutation, which confers resistance to experimental myasthenia gravis, drastically affects the epitope repertoire of murine CD4+ cells sensitized to nicotinic acetylcholine receptor. J Immunol 147:1484–1491
Berman PW, Patrick J (1980a) Experimental myasthenia gravis. A murine system. J Exp Med 151:204–223
Berman PW, Patrick J (1980b) Linkage between the frequency of muscular weakness and loci that regulate immune responsiveness in murine experimental myasthenia gravis. J Exp Med 152:507–520
Biesecker G, Gomez CM (1989) Inhibition of acute passive transfer experimental autoimmune myasthenia gravis with Fab antibody to complement C6. J Immunol 142:2654–2659
Biesecker G, Koffler D (1988) Resistance to experimental autoimmune myasthenia gravis in genetically inbred rats. Association with decreased amounts of in situ acetylcholine receptor-antibody complexes. J Immunol 140:3406–3410
Chamberlain-Banoub J, Neal JW, Mizuno M et al (2006) Complement membrane attack is required for endplate damage and clinical disease in passive experimental myasthenia gravis in Lewis rats. Clin Exp Immunol 146:278–286
Christadoss P (1988) C5 gene influences the development of murine myasthenia gravis. J Immunol 140:2589–2592
Christadoss P, Poussin M, Deng C (2000) Animal models of myasthenia gravis. Clin Immunol 94:75–87
Conti-Fine BM, Milani M, Kaminski HJ (2006) Myasthenia gravis: past, present, and future. J Clin Invest 116:2843–2854
Dau PC (1981) Response to plasmapheresis and immunosuppressive drug therapy in sixty myasthenia gravis patients. Ann NY Acad Sci 377:700–708
Deng C, Goluszko E, Tuzun E et al (2002) Resistance to experimental autoimmune myasthenia gravis in IL-6-deficient mice is associated with reduced germinal center formation and C3 production. J Immunol 169:1077–1083
Drachman DB (2003) Autonomic “myasthenia”: the case for an autoimmune pathogenesis. J Clin Invest 111:797–799
Drachman DB, Angus CW, Adams RN et al (1978) Myasthenic antibodies cross-link acetylcholine receptors to accelerate degradation. N Engl J Med 298:1116–1122
Duan RS, Adikari SB, Huang YM et al (2004) Protective potential of experimental autoimmune myasthenia gravis in Lewis rats by IL-10-modified dendritic cells. Neurobiol Dis 16:461–467
Eldefrawi ME (1978) Experimental autoimmune myasthenia gravis: the rabbit as an animal model. Fed Proc 37:2823–2827
Engel AG, Fumagalli G (1982) Mechanisms of acetylcholine receptor loss from the neuromuscular junction. Ciba Found Symp (90):197–224
Engel AG, Lambert EH, Howard FM (1977) Immune complexes (IgG and C3) at the motor end-plate in myasthenia gravis: ultrastructural and light microscopic localization and electrophysiologic correlations. Mayo Clin Proc 52:267–280
Fattorossi A, Battaglia A, Buzzonetti A et al (2005) Circulating and thymic CD4 CD25 T regulatory cells in myasthenia gravis: effect of immunosuppressive treatment. Immunology 116:134–141
Goluszko E, Deng C, Poussin MA et al (2002) Tumor necrosis factor receptor p55 and p75 deficiency protects mice from developing experimental autoimmune myasthenia gravis. J Neuroimmunol 122:85–93
Gomez CM, Richman DP (1983) Anti-acetylcholine receptor antibodies directed against the alpha-bungarotoxin binding site induce a unique form of experimental myasthenia. Proc Natl Acad Sci USA 80:4089–4093
Gomez AM, Van Den Broeck J, Vrolix K et al (2010) Antibody effector mechanisms in myasthenia gravis-pathogenesis at the neuromuscular junction. Autoimmunity 43:353–370
Gomez AM, Vrolix K, Martinez–Martinez P et al (2011) Proteasome inhibition with bortezomib depletes plasma cells and autoantibodies in experimental autoimmune myasthenia gravis. J Immunol 186:2503–2513
Hill M, Moss P, Wordsworth P et al (1999) T cell responses to D-penicillamine in drug-induced myasthenia gravis: recognition of modified DR1:peptide complexes. J Neuroimmunol 97:146–153
Hoch W, McConville J, Helms S et al (2001) Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 7:365–368
Hoedemaekers AC, Verschuuren JJ, Spaans F et al (1997a) Age-related susceptibility to experimental autoimmune myasthenia gravis: immunological and electrophysiological aspects. Muscle Nerve 20:1091–1101
Hoedemaekers AC, van Breda Vriesman PJ, De Baets MH (1997b) Myasthenia gravis as a prototype autoimmune receptor disease. Immunol Res 16:341–354
Im SH, Barchan D, Fuchs S et al (1999) Suppression of ongoing experimental myasthenia by oral treatment with an acetylcholine receptor recombinant fragment. J Clin Invest 104:1723–1730
Janssen SP, Phernambucq M, Martinez–Martinez P et al (2008) Immunosuppression of experimental autoimmune myasthenia gravis by mycophenolate mofetil. J Neuroimmunol 201–202:111–120
Kaminski HJ, Kusner LL, Richmonds C et al (2006) Deficiency of decay accelerating factor and CD59 leads to crisis in experimental myasthenia. Exp Neurol 202:287–293
Karachunski PI, Ostlie NS, Okita DK et al (1999) Interleukin-4 deficiency facilitates development of experimental myasthenia gravis and precludes its prevention by nasal administration of CD4+ epitope sequences of the acetylcholine receptor. J Neuroimmunol 95:73–84
Karachunski PI, Ostlie NS, Monfardini C et al (2000) Absence of IFN-gamma or IL-12 has different effects on experimental myasthenia gravis in C57BL/6 mice. J Immunol 164:5236–5244
Karussis DM, Lehmann D, Brenner T et al (1994) Immunomodulation of experimental autoimmune myasthenia gravis with linomide. J Neuroimmunol 55:187–193
Kong QF, Sun B, Bai SS et al (2009a) Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGF-beta. J Neuroimmunol 207:83–91
Kong QF, Sun B, Wang GY et al (2009b) BM stromal cells ameliorate experimental autoimmune myasthenia gravis by altering the balance of Th cells through the secretion of IDO. Eur J Immunol 39:800–809
Lennon VA, Lindstrom JM, Seybold ME (1975) Experimental autoimmune myasthenia: a model of myasthenia gravis in rats and guinea pigs. J Exp Med 141:1365–1375
Lennon VA, Seybold ME, Lindstrom JM et al (1978) Role of complement in the pathogenesis of experimental autoimmune myasthenia gravis. J Exp Med 147:973–983
Lennon VA, Lambert EH, Leiby KR et al (1991) Recombinant human acetylcholine receptor alpha-subunit induces chronic experimental autoimmune myasthenia gravis. J Immunol 146:2245–2248
Lin F, Kaminski HJ, Conti-Fine BM et al (2002) Markedly enhanced susceptibility to experimental autoimmune myasthenia gravis in the absence of decay-accelerating factor protection. J Clin Invest 110:1269–1274
Lindstrom J (1980) Experimental autoimmune myasthenia gravis. J Neurol Neurosurg Psychiatry 43:568–576
Lindstrom J (2008) ‘Seronegative’ myasthenia gravis is no longer seronegative. Brain 131(Pt 7):1684–1685
Lindstrom JM, Seybold ME, Lennon VA et al (1976) Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology 26:1054–1059
Link H, Xiao BG (2001) Rat models as tool to develop new immunotherapies. Immunol Rev 184:117–128
Liu R, Hao J, Dayao CS et al (2009) T-bet deficiency decreases susceptibility to experimental myasthenia gravis. Exp Neurol 220:366–373
Liu R, Zhou Q, La Cava A et al (2010) Expansion of regulatory T cells via IL-2/anti-IL-2 mAb complexes suppresses experimental myasthenia. Eur J Immunol 40:1577–1589
Losen M, Stassen MH, Martinez–Martinez P et al (2005) Increased expression of rapsyn in muscles prevents acetylcholine receptor loss in experimental autoimmune myasthenia gravis. Brain 128(Pt 10):2327–2337
Luo J, Taylor P, Losen M et al (2009) Main immunogenic region structure promotes binding of conformation-dependent myasthenia gravis autoantibodies, nicotinic acetylcholine receptor conformation maturation, and agonist sensitivity. J Neurosci 29:13898–13908
Mantegazza R, Bonanno S, Camera G et al (2011) Current and emerging therapies for the treatment of myasthenia gravis. Neuropsychiatr Dis Treat 7:151–160
Mareska M, Gutmann L (2004) Lambert-Eaton myasthenic syndrome. Semin Neurol 24:149–153
Martinez–Martinez P, Losen M, Duimel H et al (2007) Overexpression of rapsyn in rat muscle increases acetylcholine receptor levels in chronic experimental autoimmune myasthenia gravis. Am J Pathol 170:644–657
Martino G, DuPont BL, Wollmann RL et al (1993) The human-severe combined immunodeficiency myasthenic mouse model: a new approach for the study of myasthenia gravis. Ann Neurol 34:48–56
Matsui N, Nakane S, Saito F et al (2010) Undiminished regulatory T cells in the thymus of patients with myasthenia gravis. Neurology 74:816–820
Meinl E, Klinkert WE, Wekerle H (1991) The thymus in myasthenia gravis. Changes typical for the human disease are absent in experimental autoimmune myasthenia gravis of the Lewis rat. Am J Pathol 139:995–1008
Melms A, Luther C, Stoeckle C et al (2006) Thymus and myasthenia gravis: antigen processing in the human thymus and the consequences for the generation of autoreactive T cells. Acta Neurol Scand Suppl 183:12–13
Meriggioli MN, Sanders DB (2009) Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol 8:475–490
Milani M, Ostlie N, Wu H et al (2006) CD4+ T and B cells cooperate in the immunoregulation of experimental autoimmune myasthenia gravis. J Neuroimmunol 179:152–162
Moiola L, Galbiati F, Martino G et al (1998) IL-12 is involved in the induction of experimental autoimmune myasthenia gravis, an antibody-mediated disease. Eur J Immunol 28:2487–2497
Morgan BP, Chamberlain-Banoub J, Neal JW et al (2006) The membrane attack pathway of complement drives pathology in passively induced experimental autoimmune myasthenia gravis in mice. Clin Exp Immunol 146:294–302
Mu L, Sun B, Kong Q et al (2009) Disequilibrium of T helper type 1, 2 and 17 cells and regulatory T cells during the development of experimental autoimmune myasthenia gravis. Immunology 128(1 suppl):e826–e836
Nessi V, Nava S, Ruocco C et al (2010) Naturally occurring CD4+ CD25+ regulatory T cells prevent but do not improve experimental myasthenia gravis. J Immunol 185:5656–5667
Newsom-Davis J, Willcox N, Calder L (1981) Thymus cells in myasthenia gravis selectively enhance production of anti-acetylcholine-receptor antibody by autologous blood lymphocytes. N Engl J Med 305:1313–1318
Ostlie N, Milani M, Wang W et al (2003) Absence of IL-4 facilitates the development of chronic autoimmune myasthenia gravis in C57BL/6 mice. J Immunol 170:604–612
Patrick J, Lindstrom J (1973) Autoimmune response to acetylcholine receptor. Science 180:871–872
Penn AS, Low BW, Jaffe IA et al (1998) Drug-induced autoimmune myasthenia gravis. Ann NY Acad Sci 841:433–449
Pestronk A, Drachman DB, Teoh R et al (1983) Combined short-term immunotherapy for experimental autoimmune myasthenia gravis. Ann Neurol 14:235–241
Piddlesden SJ, Jiang S, Levin JL et al (1996) Soluble complement receptor 1 (sCR1) protects against experimental autoimmune myasthenia gravis. J Neuroimmunol 71:173–177
Poussin MA, Goluszko E, David CS et al (2001) HLA-DQ6 transgenic mice resistance to experimental autoimmune myasthenia gravis is linked to reduced acetylcholine receptor-specific IFN-gamma, IL-2 and IL-10 production. J Autoimmun 17:175–180
Qi H, Tuzun E, Allman W et al (2008) C5a is not involved in experimental autoimmune myasthenia gravis pathogenesis. J Neuroimmunol 196:101–106
Rodgaard A, Nielsen FC, Djurup R et al (1987) Acetylcholine receptor antibody in myasthenia gravis: predominance of IgG subclasses 1 and 3. Clin Exp Immunol 67:82–88
Roncarolo MG, Levings MK, Traversari C (2001) Differentiation of T regulatory cells by immature dendritic cells. J Exp Med 193:F5–F9
Ruff RL, Lennon VA (1998) End-plate voltage-gated sodium channels are lost in clinical and experimental myasthenia gravis. Ann Neurol 43:370–379
Russel WMS, Burch RL (1959) The principles of humane experimental technique. Special edition published by Universities Federation for Animal Welfare (UFAW), 1992 edn. Methuen & Co., London
Sahashi K, Engel AG, Lambert EH et al (1980) Ultrastructural localization of the terminal and lytic ninth complement component (C9) at the motor end-plate in myasthenia gravis. J Neuropathol Exp Neurol 39:160–172
Sakaguchi S (2004) Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 22:531–562
Sanders DB, Evoli A (2010) Immunosuppressive therapies in myasthenia gravis. Autoimmunity 43:428–435
Schönbeck S, Padberg F, Hohlfeld R et al (1992) Transplantation of thymic autoimmune microenvironment to severe combined immunodeficiency mice. A new model of myasthenia gravis. J Clin Invest 90:245–250
Sheng JR, Li L, Ganesh BB et al (2006) Suppression of experimental autoimmune myasthenia gravis by granulocyte-macrophage colony-stimulating factor is associated with an expansion of FoxP3+ regulatory T cells. J Immunol 177:5296–5306
Slater CR, Young C, Wood SJ et al (1997) Utrophin abundance is reduced at neuromuscular junctions of patients with both inherited and acquired acetylcholine receptor deficiencies. Brain 120(Pt 9):1513–1531
Soltys J, Kusner LL, Young A et al (2009) Novel complement inhibitor limits severity of experimentally myasthenia gravis. Ann Neurol 65:67–75
Tarrab-Hazdai R, Aharonov A, Silman I et al (1975) Experimental autoimmune myasthenia induced in monkeys by purified acetylcholine receptor. Nature 256:128–130
Toro-Goyco E, Cora EM, Kessler MJ et al (1986) Induction of experimental myasthenia gravis in rhesus monkeys: a model for the study of the human disease. PR Health Sci J 5:13–18
Toyka KV, Brachman DB, Pestronk A et al (1975) Myasthenia gravis: passive transfer from man to mouse. Science 190:397–399
Tzartos S, Hochschwender S, Vasquez P et al (1987) Passive transfer of experimental autoimmune myasthenia gravis by monoclonal antibodies to the main immunogenic region of the acetylcholine receptor. J Neuroimmunol 15:185–194
Tzartos SJ, Kokla A, Walgrave SL et al (1988) Localization of the main immunogenic region of human muscle acetylcholine receptor to residues 67–76 of the alpha subunit. Proc Natl Acad Sci USA 85:2899–2903
Ubiali F, Nava S, Nessi V et al (2008) Pixantrone (BBR2778) reduces the severity of experimental autoimmune myasthenia gravis in Lewis rats. J Immunol 180:2696–2703
van der Neut Kolfschoten M, Schuurman J, Losen M et al (2007) Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 317:1554–1557
Vincent A (1991) Autoimmunity to acetylcholine receptors in myasthenia gravis. Biochem Soc Trans 19:180–183
Vincent A (1994) AChR from cell line TE671 cannot replace human muscle AChR in the conventional diagnostic immunoprecipitation RIA. J Neuroimmunol 53:115
Vincent A (2002) Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol 2:797–804
Wang ZY, Karachunski PI, Howard JF et al (1999) Myasthenia in SCID mice grafted with myasthenic patient lymphocytes: role of CD4+ and CD8+ cells. Neurology 52:484–497
Wang W, Ostlie NS, Conti-Fine BM et al (2004) The susceptibility to experimental myasthenia gravis of STAT6−/− and STAT4−/− BALB/c mice suggests a pathogenic role of Th1 cells. J Immunol 172:97–103
Wood SJ, Slater CR (2001) Safety factor at the neuromuscular junction. Prog Neurobiol 64:393–429
Xiao BG, Duan RS, Link H et al (2003) Induction of peripheral tolerance to experimental autoimmune myasthenia gravis by acetylcholine receptor-pulsed dendritic cells. Cell Immunol 223:63–69
Xiao BG, Duan RS, Zhu WH et al (2006) The limitation of IL-10-exposed dendritic cells in the treatment of experimental autoimmune myasthenia gravis and myasthenia gravis. Cell Immunol 241:95–101
Yang H, Goluszko E, David C et al (2002) Mapping myasthenia gravis-associated T cell epitopes on human acetylcholine receptors in HLA transgenic mice. J Clin Invest 109:1111–1120
Yang H, Zhang Y, Wu M et al (2010) Suppression of ongoing experimental autoimmune myasthenia gravis by transfer of RelB-silenced bone marrow dentritic cells is associated with a change from a T helper Th17/Th1 to a Th2 and FoxP3+ regulatory T-cell profile. Inflamm Res 59:197–205
Yarilin D, Duan R, Huang YM et al (2002) Dendritic cells exposed in vitro to TGF-beta1 ameliorate experimental autoimmune myasthenia gravis. Clin Exp Immunol 127:214–219
Zhang GX, Xiao BG, Bai XF et al (1999) Mice with IFN-gamma receptor deficiency are less susceptible to experimental autoimmune myasthenia gravis. J Immunol 162:3775–3781
Zhou Y, Gong B, Lin F et al (2007) Anti-C5 antibody treatment ameliorates weakness in experimentally acquired myasthenia gravis. J Immunol 179:8562–8567
Acknowledgments
We regret that the essential work of many investigators and colleagues could not be included in this review due to space constraints.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Baggi, F., Antozzi, C., Toscani, C. et al. Acetylcholine Receptor-Induced Experimental Myasthenia Gravis: What Have We Learned from Animal Models After Three Decades?. Arch. Immunol. Ther. Exp. 60, 19–30 (2012). https://doi.org/10.1007/s00005-011-0158-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-011-0158-6