Abstract
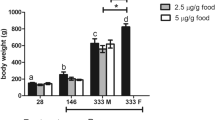
17α-ethinylestradiol (EE2) is a potent synthetic estrogen that is routinely detected in aquatic ecosystems and exhibits estrogenic activity. Acute and chronic toxicity have been described for oviparous and ovoviviparous fish species; however, no information is available on the impacts of EE2 on viviparous, matrotrophic fish despite their ecological importance. The present study investigated the consequences of long-term EE2 exposure in the least killifish (Heterandria formosa). Effects on growth, time-to-sexual maturity, fecundity, and offspring survival were examined in an 8-month, life-cycle experiment. Starting as 0–6-day-old fish, least killifish were continuously exposed to EE2 at nominal concentrations of 0, 5, or 25 ng/L (measured concentrations averaged 0, 4.3, and 21.5 ng/L respectively). In the F0 generation, EE2-exposure did not affect survival but resulted in increased time-to-sexual maturity and a sex-dependent effect on size; female standard length was reduced while male standard length was increased. This caused the ordinarily larger females and smaller males to become more similar in size. Condition factor was reduced for both sexes. Fecundity was reduced by 50% and 75% at 5 and 25 ng/L EE2-exposure respectively. Continued EE2-exposure in the F1 generation resulted in significantly reduced survival. These results suggest that despite their matrotrophic development, these fish experience delayed development and reduced reproductive success from EE2-exposure and that effects appear to intensify in the second generation.







Similar content being viewed by others
Data Availability
Data pertaining to this manuscript are available, upon request, from the lead author (LMJ).
References
Abramoff M, Magalhaes P, Ram S (2004) Image processing with image. J Biophotonics 11:36–42
Al-Ansari A, Saleem A, Kimpe L, Sherry J, McMaster M, Trudeau V, Blais J (2010) Bioaccumulation of the pharmaceutical 17α-ethinylestradiol in shorthead redhorse suckers (Moxostoma macrolepidotum) from the St. Clair River, Canada. Environ Pollut 158:2566–2571
Balch G, Mackenzie C, Metcalfe C (2004) Alterations to gonadal development and reproductive success in Japanese medaka (Oryzias latipes) exposed to 17α-ethinylestradiol. Environ Toxicol Chem 23:782–791
Barel-Cohen K, Shore L, Shemesh M, Wenzel A, Mueller J, Kronfeld-Schor N (2006) Monitoring of natural and synthetic hormones in a polluted river. J Environ Manag 78:16–23
Belfroid A, Van der Horst A, Vethaak A, Schafer A, Rijs G, Wegener J, Cofino W (1999) Analysis and occurrence of estrogenic hormones and their glucuronides in surface water and waste water in The Netherlands. Sci Total Environ 225:101–108
Belk M, Lydeard C (1994) Effect of Gambusia holbrooki on a similar-sized, syntopic Poeciliid, Heterandria formosa: competitor or predator? Copeia 1994:296–302
Bergman Å, Heindel J, Jobling S, Kidd K, Zoeller R (2012) State-of-the-science of endocrine disrupting chemicals, 2012: an assessment of the state of the science of endocrine disruptors prepared by a group of experts for the United Nations Environment Programme and World Health Organization. Toxicol Lett 211:S3
Bhandari R, Saal F, Tillitt D (2015) Transgenerational effects from early developmental exposures to bisphenol A or 17α-ethinylestradiol in medaka, Oryzias latipes. Sci Rep. https://doi.org/10.1038/srep09303
Bizarro C, Ros O, Vallejo A, Prieto A, Etxebarria N, Cajaraville M, Ortiz-Zarragoitia M (2014) Intersex condition and molecular markers of endocrine disruption in relation with burdens of emerging pollutants in thicklip grey mullets (Chelon labrosus) from Basque estuaries (South-East Bay of Biscay). Mar Environ Res 96:19–28
Blackwell B, Brown M, Willis D (2000) Relative weight (Wr) status and current use in Fisheries assessment and management. Rev Fish Sci 8:1–44
Buchberger W (2007) Novel analytical procedures for screening of drug residues in water, waste water, sediment and sludge. Anal Chim Acta 593:129–139
Cargouet M, Perdiz D, Mouatassim-Souali A, Tamisier-Karolak S, Levi Y (2004) Assessment of river contamination by estrogenic compounds in Paris area (France). Sci Total Environ 324:55–66
Chen C, When T, Wang G, Cheng H, Lin Y, Lien G (2010) High estrogen concentrations in receiving river discharg from a concentrated livestock feedlot. Sci Total Environ 408:3223–3230
Cheong R, Henrich S, Farr J, Travis J (1984) Variation in fecundity and its relationship to body size in a population of the least killifish, Heterandria formosa (Pisces: Poeciliidae). Copeia 3:720–726
Christiansen L, Winther-Nelsen M, Helweg C (2002) Feminization of fish: the effect of estrogenic compounds and their fate in sewage treatment plants and nature (Environmental Project No. 720-2002). Danish Environmental Protection Agency, København, Denmark
Clouzot L, Marrot B, Doumenq P, Roce N (2008) 17 alpha-ethinylestradiol: an endocrine disrupter of great concern. Analytical methods and removal processes applied to water purification. A review. Environ Progr Sustain Energy 27:383–396
Constanz G (1989) Reproductive biology of the poeciliid fishes. In: Meffe GK, Snelson FF Jr (eds) Englewood Cliffs. Prentice Hall, NJ
Desbrow C, Routledge E, Brighty G, Sumpter J, Waldock M (1998) Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environ Sci Technol 32:1549–1557
Diaz-Cruz S, Barcelo’ D (2004) Occurrence and analysis of selected pharmaceuticals and metabolites as contaminants present in wastewaters, sludge and sediments. Handb Environ Chem 5:227–260
Duong C et al (2010) Estrogenic chemicals and estrogenicity in river waters of South Korea and seven Asian countries. Chemosphere 78:286–293
Dziewecyznski T, Hebert O (2013) The effects of short-term exposure to an endocrine disrupter on behavioural consistency in male juvenile and adult Siamese fishting fish. Arch Environ Contam Toxicol 64:316–326
Faulk C, Fuiman L, Thomas P (1999) Parental exposure to ortho, paradichlorodiphenyltrichloroethane impairs survival skills of Atlantic croaker (Micropogonias undulatus) larvae. Environ Toxicol Chem 18:254–262
Fonseca A, Cardoso M, Esteves V (2013) Determination of estrogens in raw and treated wastewater by high-performance liquid chromatograpy: ultraviolet detection. J Environ Anal Toxicol 4:203
Foran C, Peterson B, Benson W (2002) Transgenerational and developmental exposure of Japanese medaka (Oryzias latipes) to ethinylestradiol results in endocrine and reproductive differences in the response to ethinylestradiol as adults. J Toxicol Sci 68:389–402
Fraser E, Renton R (1940) Observation on the breeding and development of the viviparous fish Heterandria formosa. Q J Microsc Sci 81:479–520
Gadd J, Tremblay L, Northcott G (2010) Steroid estrogens, conjugated estrogens and estrogenic activity in farm dairy shed effluents. Environ Pollut 158:730–736
Gennotte V et al (2015) Brief exposure of embryos to steroids or aromatase inhibitor induces sex reversal in Nile tilapia (Oreochromis niloticus). J Exp Zool 323A:31–38
Gibson R, Smith M, Spary C, Tyler C, Hill A (2005) Mixtures of estrogenic contaminates in the bile of fish exposed to wastewater treatment works effluents. Environ Sci Technol 39:2461–2471
Hardman J, Limbird L, Molinoff P, Ruddon R, Goodman A (1996) Goodman and Gilman’s: the pharmacological basis of therapeutics. Toxicol Lett 86:1–55
Hemming J, Turner P, Brooks B, Waller W, LaPoint T (2002) Assessment of toxicity reduction in wastewater effluent flowing through a treatment wetland using Pimephales promelas, Ceriodaphnia dubia, and Vibrio fischeri. Arch Environ Contam Toxicol 42:9–16
Jackson L, Felgenhauer B, Klerks P (2018) Feminization, altered gonadal development, and liver damage in least killifish (Heterandria formosa) exposed to sublethal concentrations of 17α-ethinylestradiol. Ecotox Environ Safe 170:331–337
Johnson A (2010) Natural variations in flow are critical in determining concentrations fo point source contaminants in rivers; as estrogen example. Environ Sci Technol 44:7865–7870
Johnson A, Sumpter P (2014) Putting pharmaceuticals into the wider context of challenges to fish populations in rivers. Phil Trans R Soc B. https://doi.org/10.1098/rstb.2013.0581
Jurgens M, Holthaus K, Johnson A, Smith J (2002) The potential for estradiol and ethynylestradiol degradation in English rivers. EnvironToxicol Chem 21:480–488
Kah O, Dufour S (2011) Conserved and divergent features of reproductive neuroendocrinology in teleost fishes. In: Norris D, Lopez K (eds) Hormones and reproduction of vertebrates. Academic Press, London, pp 15–42
Kidd K, Blanchfield P, Mills K, Palace V, Evans R, Lazorchak J, Flick R (2007) Collapse of a fish population after exposure to a synthetic estrogen. PNAS 104:8897–8901
Kitamura S, Ogata H, Takashima F (1994) Activities of F-type prostaglandins as releaser sex pheromones in cobitide loach. Misgurnus anguillicaudatus Comp Biochem Physiol A 107:161–169
Kolpin D, Furlong E, Meyer M, Thurman E, Zaugg S, Barber L, Buxton H (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36:1202–1211
Kostich M, Flick R, Martinson J (2013) Comparing predicted estrogen concentrations with measurements in U.S. waters. Environ Pollut 178:271–277
Kristensen T, Baatrup E, Bayley M (2005) 17α-ethinylestradiol reduces the competitive reproductive fitness of the male guppy (Poecilia reticulate). Biol Reprod 72:150–156
Kunz P, Kienle C, Carere M, Homazava N, Kase R (2015) In vitro bioassays to screen for endocrine active pharmaceuticals in surface and waste waters. JPBA 106:107–115
Lange R et al (2001) Effects of the synthetic estrogen 17α-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environ Toxicol Chem 20:1216–1227
Lange A, Paull G, Hamilton P, Iguchi T, Tyler C (2011) Implications of persistent exposure to treated wastewater effluent for breeding in wild roach (Rutilus rutilus) populations. Environ Sci Technol 45:1673–1679
Langer R (2009) Efficacy, safety, and tolerability of low-dose hormone therapy in managing menopausal symptoms. J Am Board Fam Med 22:563–573
Larsson D, Adolfsson-Erici M, Parkkonen J, Petterson M, Berg A, Olsson P, Forlin L (1999) Ethynylestradiol: an undesired fish contraceptive. Aquat Toxicol 454:91–97
Lavelle C, Sorensen P (2011) Behavioral responses of adult male and female fathead minnows to a model estrogenic effluent and its effects on exposure regime and reproductive success. Aquat Toxicol 101:521–528
Lazorchak J, McCormick F, Henry T, Herlihy A (2003) Contamination of fish in streams of the Mid-Atlantic Region: an approach to regional indicator selection and wildlife assessment. Environ Toxicol Chem 22:545–553
Lei B, Huang S, Zhou Y, Wang D, Wang Z (2009) Levels of six estrogens in water and sediment from three rivers in Tianjin area, China. Chemosphere 76:36–42
Liu J, Wang R, Huang B, Lin C, Zhou J, Pan X (2012a) Biological effects and bioaccumulation of steroidal and phenolic endocrine disrupting chemicals in high-back crucian carp exposed to wastewater treatment plant effluents. Environ Pollut 162:325–331
Liu S, Ying G, Zhou L, Zhang R, Chen Z, Lai H (2012b) Steroids in typical swine farm and their release into the environment. Water Res 45:3754–3768
Martin S, Hitch A, Purcell K, Klerks P, Leberg P (2009) Life history variation along a salinity gradient in coastal marshes. Aquat Biol 8:15–28
Montgomery T, Brown A, Gendelman H, Ota M, Clottfelter E (2012) Exposure to 17α-ethinylestradiol decreases motility and ATP in sperm of male fighting fish Betta splendens. Environ Toxicol 29:243–252
Nash J, Van der Ven L, Brion F, Maack G, Stahlschmidt-Allner P, Tyler C (2004) Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environ Health Perspect 112:1725–1733
Nehemia A, Maganira J, Rumisha C (2012) Length-weight relationship and condition factor of talapia species grown in marine and fresh water ponds. Agric Biol J North Am 3:117–124
Nelson E, Do H, Lewis R, Carr S (2011) Diurnal variability of pharmaceutical, personal care product, estrogen and alkylphenol concentrations in effluent from a tertiary wastewater treatment facility. Environ Sci Technol 45:1228–1234
Pojana G, Gomiero A, Jonkers N, Marcomini A (2007) Natural and synthetic endocrine disrupting compounds (EDCs) in water, sediment and biota of a coastal lagoon. Environ Int 33:929–936
Reznick D, Callahan H, Llauredo R (1996) Maternal effects on offspring quality in poeciliid fishes. Am Zool 36:147–156
Rolland R (2000) Ecoepidemiology of the effects of pollution on reproduction and survival of early life stages in teleosts. Fish Fish 1:41–72
Rosen D, Bailey R (1963) The poeciliid fishes (Cyprinodontiformes), their structure, zoo-geography, and systematics. B Am Mus Nat Hist 126:1–176
Rosen D, Gordon M (1953) Functional anatomy and evolution of male genitalia in poeciliid fishes. Zoologica 38:1–47
Saaristo M, Craft J, Lehtonen K, Lindstrom K (2009) Sand goby (Pomatoschistus minutus) males exposed to endocrine disrupting chemical fail in nest and mate competition. Horm Behav 56:315–321
Salla R et al (2016) Impact of an environmental relevant concentration of 17α-ethinylestradiol on the cardiac function of bullfrog tadpoles. Chemosphere 144:1862–1868
Sarat M, Rambabu C (2012) A validated simultaneous RP-HPLC method for determination of desogestrel and ethinyl estradiol tablets. Int J Pharm Pharm Sci 4:115–119
Scholz S, Gutzeit H (2000) 17-a-ethynylestradiol affects reproduction, sexual differentiation and aromatase gene expression of the medaka (Oryzias latipes). Aquat Toxicol 50:363–373
Schultz I, Skillman A, Nicolas J, Cyr D, Nagler J (2003) Short-term exposure to 17a-ethynylestradiol decreases the fertility of sexually maturing male rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 22:1272–1280
Schwindt A, Winkelman D, Keteles K, Murphy M, Vajda A (2014) An environmental oestrogen disrupts fish population dynamics through direct and transgenerational effects on survival and fecundity. J Appl Ecol 51:582–591
Scrimshaw N (1944) Embryonic growth in the viviparous poeciliid, Heterandria formosa. Biol Bull 87:37–51
Segner H, Navas J, Schafers C, Wenzel A (2003) Potencies of estrogenic compounds in in vitro screening assays and in life cycle tests with zebrafish in vivo. Ecotox Environ Safe 54:315–322
Shifren J, Schiff I (2010) Role of hormone therapy in the management of menopause. Obstet Gynecol 115:839–855
Silva P et al (2012) Testing the effects of ethinylestradiol and of an environmentally relevant mixture of xenoestrogens as found in the Douro River (Portugal) on the maturatioin of fish gonads: a stereological study using the zebrafish (Danio rerio) as model. Aquat Toxicol 124–125:1–10
Snyder S, Keith T, Verbrugge D, Snyder E, Gross T, Kannan K, Giesy J (1999) Analytical methods for detection of selected estrogenic compounds in aqueous mixtures. Environ Sci Technol 33:2814–2820
Sole’ M, Raldua D, Piferrer F, Barcelo’ D, Porte C (2003) Feminization of wild carp, Cyprinus carpio, in a polluted environment: plasma steroid hormones, gonadal morphology and xenobiotic metabolizing system. Comp Biochem Phys C 136:145–156
Sorensen P, Goetz F (1993) Pheromonal and reproductive function of F-prostaglandins and their metabolites in teleost fish. J Lipid Mediators 6:385–393
Stacey N, Peter R (1979) Central action of prostaglandins in spawning behavior of female goldfish. Physiol Behav 22:1191–1196
Sumpter J (2005) Endocrine disrupters in the aquatic environment: an overview. Acta Hydroch Hydrob 33:9–16
Sun L, Yong W, Chu X, Lin J (2009) Simultaneous determination fo 15 steroidal oral contraceptives in water using splid-phase disk extraction followed by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1216:5416–5423
Tamschick S et al (2016) Sex reversal assessments reveal different vulnerability to endocrine disruption between deeply diverged anuran lineages. Sci Reg. https://doi.org/10.1038/srep23825
Ternes T, Stumpf M, Mueller J, Haberer K, Wilken R, Servos M (1999) Behavior and occurrence of estrogens in municipal sewage treatment plants-I. Investigations in Germany, Canada and Brazil. Sci Total Environ 225:81–90
Teta C, Naik Y (2016) Vitellogenin induction and reduced fecundity in zebrafish exposed to effluents from the city of Bulawayo, Zimbabwe. Chemosphere 167:282–290
Travis J, Farr J, Henrich S, Cheong R (1987) Testing theories of clutch overlap with the reproductive ecology of Heterandria formosa. Ecology 68:611–623
Trexler J, Travis J, Trexler M (1990) Phenotypic plasticity in the sailfin molly, Poecilia latipinna (Pisces: Poeciliidae). II. Laboratory experiment. Evolution 44:157–167
Trudeau V, Metcalfe C, Mimeault C, Moon T (2005) Pharmaceuticals in the environment: drugged fish? Biochem Mol Biol Fishes 6:475–493
Turner C (1937) Reproductive cycles and superfetation in poeciliid fishes. Biol Bull 72:145–164
Van Aerle R, Nolanusan M, Jobling S, Christiansen L, Sumpter J, Tyler C (2001) Sexual disruption in a second species of wild cyprinid fish (the gudgeon, Gobio gobio) in United Kingdom freshwaters. Environ Toxicol Chem 20:2841–2847
Van den Belt K, Verheyen R, Witters H (2003) Effects of 17a-ethinylestradiol in a partial life-cycle test with zebrafish (Danio rerio): effects on growth, gonads and female reproductive success. Sci Total Environ 309:127–137
Versonnen B, Arijs K, Verslycke T, Lema W, Janssen C (2003) In vitro and in vivo estrogenicity and toxicity of o-, m-, and p-dichlorobenzene. Environ Toxicol Chem 22:329–335
Vieno N, Harkki H, Tuhkanen T, Kronberg L (2007) Occurrence of pharmaceuticals in river water and their elimination in a pilot-scale drinking water treatment plant. Environ Sci Technol 41:5077–5084
Wang L et al (2011) Assessing estrogenic activity in surface water and sediment of the Liao River systems in northeast China using combined chemical and biological tools. Environ Pollut 159:148–156
Williams R (2005) Human pharmaceuticals: assessing the impacts on aquatic ecosystems. Alan Press/ACG Publishing, Lawrence
World Health Organization (WHO) (2012) State of the science of endocrine disrupting chemicals- 2012. World Health Organization, International Programme on Chemical Safety, Geneva
Xu J, Wu L, Chen W, Jiang P, Chang A (2009) Pharmaceuticals and personal care products (PPCPs) and endocrine disrupting compounds (EDCs) in runoff from a potato field irrigated with treated wastewater in Southern California. J Health Sci 55:306–310
Yan Z, Lu G, Liu J, Jin S (2012) An integrated assessment of estrogenic contamination and feminization risk in fish in Taihu Lake, China. Ecotox Environ Safe 84:334–340
Yang Y, Gray J, Furlong E, Davis J, ReVello R, Borch T (2012) Steroid hormone runofff from agricultural test plots applied with municipal biosolids. Environ Sci Technol 45:2746–2754
Ying G, Rai K, Ying-Jun R (2002) Occurrence and fate of hormone steroids in the environment. Environ Int 28:545–551
Ying G, Kookana R, Kumar A, Mortimer M (2009) Occurrence and implications of estrogens and xenoestrogens in sewage effluents and receiving waters from South East Queensland. Sci Total Environ 407:5147–5155
Zhang Z, Feng Y, Gao P, Wang C, Ren N (2011) Occurrence and removal efficiencies of eight EDCs and estrogenicity in a STP. J Environ Monit 13:1366–1373
Zou Y, Zhang K, Zhou S (2013) Determination of estrogenic steroids and microbial and photochemical degradation of 17α-ethinylestradiol (EE2) in lake surface water: a case study. Environ Sci Process Impacts 15:1529–1535
Acknowledgements
The authors greatly appreciate the editorial comments from S. Duke-Sylvester, B. Felgenhauer, P. Leberg, and J. McLachlan, and the logistical support provided by J. Adeyemi, S. Volt, J. Hill, E. Blankson, A. Cazan, A. Oguma, A. Kascak, A. Pant, Q. Jackson, L. Carrier, and the staff of the UL Lafayette Ecology Center.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jackson, L.M., Klerks, P.L. Impact of Long-Term Exposure to 17α-Ethinylestradiol in the Live-Bearing Fish Heterandria formosa. Arch Environ Contam Toxicol 77, 51–61 (2019). https://doi.org/10.1007/s00244-019-00600-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-019-00600-5