Abstract
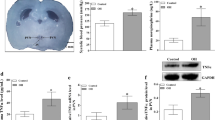
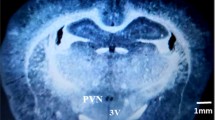
Our previous study showed that the adipose afferent reflex (AAR) induced by chemical stimulation of white adipose tissue (WAT) increased sympathetic outflow and blood pressure. We also found that pro-inflammatory cytokines (PICs) in the hypothalamic paraventricular nucleus (PVN) potentiate the cardiac sympathetic afferent reflex in rats. However, the role of PICs in the PVN in regulating the AAR is still not clear. This study determined whether PICs in the PVN mediate the AAR in rats. The AAR was evaluated based on renal sympathetic nerve activity and mean arterial blood pressure in response to capsaicin injection into inguinal WAT (iWAT). PIC levels were measured by ELISA. PVN microinjection with the PICs tumor necrosis factor (TNF)–α or interleukin (IL)-1β enhanced the AAR in a dose-dependent manner. Furthermore, pretreatment via the bilateral microinjection of the TNF-α-blocker etanercept or IL-1β blocker IL-1ra into the PVN attenuated the AAR. In rats pretreated with TNF-α or IL-1β, a sub-response dose of angiotensin II (Ang II) significantly enhanced the AAR. Moreover, delivery of the angiotensin II type 1(AT1) receptor antagonist losartan into the PVN attenuated the effects of TNF-α or IL-1β on the AAR. In addition, stimulating either iWAT or retroperitoneal WAT with capsaicin increased TNF-α or IL-1β levels in the PVN, but the injection of capsaicin into the jugular vein, skeletal muscle, and skin had no effects on TNF-α or IL-1β levels in the PVN. These results suggest that TNF-α or IL-1β and Ang II in the PVN synergistically enhance the AAR in rats.





Similar content being viewed by others
References
Badoer E (2010) Role of the hypothalamic PVN in the regulation of renal sympathetic nerve activity and blood flow during hyperthermia and in heart failure. Am J Physiol Ren Physiol 298:F839–F846. https://doi.org/10.1152/ajprenal.00734.2009
Berthoud HR, Powley TL (1993) Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J Auton Nerv Syst 42(2):153–169. https://doi.org/10.1016/0165-1838(93)90046-w
Bartness TJ, Kay SC, Shi H, Bowers RR, Foster MT (2005) Brain-adipose tissue cross talk. Proc Nutr Soc 64:53–64. https://doi.org/10.1079/PNS2004409
Bartness TJ, Song CK (2007) Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res 48:1655–1672. https://doi.org/10.1194/jlr.R700006-JLR200
Biancardi VC, Bomfim GF, Reis WL, Al-Gassimi S, Nunes KP (2017) The interplay between angiotensin II, TLR4 and hypertension. Pharmacol Res 120:88–96. https://doi.org/10.1016/j.phrs.2017.03.017
Blaszkiewicz M, Willows JW, Johnson CP, Townsend KL (2019) The importance of peripheral nerves in adipose tissue for the regulation of energy balance. Biology (Basel) 8:10. https://doi.org/10.3390/biology8010010
Cartmell T, Luheshi GN, Rothwell NJ (1999) Brain sites of action of endogenous interleukin-1 in the febrile response to localized inflammation in the rat. J Physiol 518(Pt 2):585–594. https://doi.org/10.1111/j.1469-7793.1999.0585p.x
Chen QH, Toney GM (2001) AT(1)-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Phys Regul Integr Comp Phys;281(6):R1844–R1853. https://doi.org/10.1152/ajpregu.2001.281.6.R1844
Dampney RA, Michelini LC, Li DP, Pan HL (2018) Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am J Physiol Heart Circ Physiol 315:H1200–H1214. https://doi.org/10.1152/ajpheart.00216.2018
Dange RB, Agarwal D, Teruyama R, Francis J (2015) Toll-like receptor 4 inhibition within the paraventricular nucleus attenuates blood pressure and inflammatory response in a genetic model of hypertension. J Neuroinflammation 12:31. https://doi.org/10.1186/s12974-015-0242-7
Ding L, Zhang LL, Gao R, Chen D, Wang JJ, Gao XY, Kang YM, Zhu GQ (2013) Superoxide anions in paraventricular nucleus modulate adipose afferent reflex and sympathetic activity in rats. PLoS One 8:e83771. https://doi.org/10.1371/journal.pone.0083771
Gong X, Hu H, Qiao Y, Xu P, Yang M, Dang R, Han W, Guo Y, Chen D, Jiang P (2019) The involvement of renin-angiotensin system in lipopolysaccharide-induced behavioral changes, neuroinflammation, and disturbed insulin signaling. Front Pharmacol 10:318. https://doi.org/10.3389/fphar.2019.00318
Haspula D, Clark MA (2018) Neuroinflammation and sympathetic overactivity: mechanisms and implications in hypertension. Auton Neurosci 210:10–17. https://doi.org/10.1016/j.autneu.2018.01.002
Jiang Y, Deacon R, Anthony DC, Campbell SJ (2008) Inhibition of peripheral TNF can block the malaise associated with CNS inflammatory diseases. Neurobiol Dis 32(1):125–132. https://doi.org/10.1016/j.nbd.2008.06.017
Kang YM, Yang Q, Yu XJ, Qi J, Zhang Y, Li HB, Su Q, Zhu GQ (2014) Hypothalamic paraventricular nucleus activation contributes to neurohumoral excitation in rats with heart failure. Regen Med Res 2:2. https://doi.org/10.1186/2050-490X-2-2
Lori A, Perrotta M, Lembo G, Carnevale D (2017) The spleen: a hub connecting nervous and immune systems in cardiovascular and metabolic diseases. Int J Mol Sci Jun 18:1216. https://doi.org/10.3390/ijms18061216
Lu P, Jiang SJ, Pan H, Xu AL, Wang GH, Ma CL, Shi Z (2018) Short hairpin RNA interference targeting interleukin 1 receptor type I in the paraventricular nucleus attenuates hypertension in rats. Pflugers Arch 470:439–448. https://doi.org/10.3390/ijms18061216
Niijima A (1999) Reflex effects from leptin sensors in the white adipose tissue of the epididymis to the efferent activity of the sympathetic and vagus nerve in the rat. Neurosci Lett 262:125–128. https://doi.org/10.1016/s0304-3940(99)00054-3
Niijima A (1998) Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. J Auton Nerv Syst 73:19–25. https://doi.org/10.1016/S0165-1838(98)00109-X
Qi J, Zhang DM, Suo YP, Song XA, Yu XJ, Elks C, Lin YX, Xu YY, Zang WJ, Zhu Z, Kang YM (2013) Renin-angiotensin system modulates neurotransmitters in the paraventricular nucleus and contributes to angiotensin II-induced hypertensive response. Cardiovasc Toxicol 13:48–54. https://doi.org/10.1007/s12012-012-9184-9
Qin C, Li J, Tang K (2018) The Paraventricular nucleus of the hypothalamus: development, function, and human diseases. Endocrinology 159:3458–3472. https://doi.org/10.1210/en.2018-00453
Ryu V, Bartness TJ (2014) Short and long sympathetic-sensory feedback loops in white fat. Am J Phys Regul Integr Comp Phys 306:R886–R900. https://doi.org/10.1152/ajpregu.00060.2014
Shen XZ, Li Y, Li L, Shah KH, Bernstein KE, Lyden P, Shi P (2015) Microglia participate in neurogenic regulation of hypertension. Hypertension 66:309–316. https://doi.org/10.1161/HYPERTENSIONAHA.115.05333
Shi Z, Chen WW, Xiong XQ, Han Y, Zhou YB, Zhang F, Gao XY, Zhu GQ (2012) Sympathetic activation by chemical stimulation of white adipose tissues in rats. J Appl Physiol 112:1008–1014. https://doi.org/10.1152/japplphysiol.01164.2011
Shi Z, Gan XB, Fan ZD, Zhang F, Zhou YB, Gao XY, De W, Zhu GQ (2011) Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxford) 203:289–297. https://doi.org/10.1111/j.1748-1716.2011.02313.x
Shi Z, Jiang SJ, Wang GH, Xu AL, Guo L (2014) Pro-inflammatory cytokines in paraventricular nucleus mediate the cardiac sympathetic afferent reflex in hypertension. Auton Neurosci 186:54–61. https://doi.org/10.1016/j.autneu.2014.10.001
Shi Z, Wang YF, Wang GH, Wu YL, Ma CL (2016) Paraventricular nucleus is involved in the central pathway of adipose afferent reflex in rats. Can J Physiol Pharmacol 94:534–541. https://doi.org/10.1139/cjpp-2015-0097
Song CK, Schwartz GJ, Bartness TJ (2009) Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Phys Regul Integr Comp Phys 296:R501–R511. https://doi.org/10.1152/ajpregu.90786.2008
Su Q, Qin DN, Wang FX, Ren J, Li HB, Zhang M, Yang Q, Miao YW, Yu XJ, Qi J, Zhu Z, Zhu GQ, Kang YM (2014) Inhibition of reactive oxygen species in hypothalamic paraventricular nucleus attenuates the renin-angiotensin system and proinflammatory cytokines in hypertension. Toxicol Appl Pharmacol 276:115–120. https://doi.org/10.1016/j.taap.2014.02.002
Tanida M, Iwashita S, Ootsuka Y, Terui N, Suzuki M (2000) Leptin injection into white adipose tissue elevates renal sympathetic nerve activity dose-dependently through the afferent nerves pathway in rats. Neurosci Lett 293:107–110. https://doi.org/10.1016/s0304-3940(00)01490-7
Trayhurn P, Beattie JH (2001) Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc 60:329–339. https://doi.org/10.1079/PNS200194
Vazquez-Vela ME, Torres N, Tovar AR (2008) White adipose tissue as endocrine organ and its role in obesity. Arch Med Res 39:715–728. https://doi.org/10.1016/j.arcmed.2008.09.005
Wakita T, Shintani F, Yagi G, Asai M, Nozawa S (2001) Combination of inflammatory cytokines increases nitrite and nitrate levels in the paraventricular nucleus of conscious rats. Brain Res 905:12–20. https://doi.org/10.1016/s0006-8993(01)02346-0
Winklewski PJ, Radkowski M, Wszedybyl-Winklewska M, Demkow U (2015) Brain inflammation and hypertension: the chicken or the egg? J Neuroinflammation 12:85. https://doi.org/10.1186/s12974-015-0306-8
Xiong XQ, Chen WW, Han Y, Zhou YB, Zhang F, Gao XY, Zhu GQ (2012) Enhanced adipose afferent reflex contributes to sympathetic activation in diet-induced obesity hypertension. Hypertension 60:1280–1286. https://doi.org/10.1161/HYPERTENSIONAHA.112.198002
Yu Y, Wei SG, Weiss RM, Felder RB (2018) Angiotensin II type 1a receptors in the subfornical organ modulate neuroinflammation in the hypothalamic paraventricular nucleus in heart failure rats. Neuroscience 381:46–58. https://doi.org/10.1016/j.neuroscience.2018.04.012
Zhang ZH, Francis J, Weiss RM, Felder RB (2002) The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol 283:H423–H433. https://doi.org/10.1152/ajpheart.00685.2001
Acknowledgments
The authors would like to thank Professor Guo-qing Zhu, Key Laboratory of Cardiovascular Disease and Molecular Intervention, Department of Physiology, Nanjing Medical University, for his technical assistance, and Peng Li and Wei-wei Chen for their help with experiments. We would also like to thank Editage (www.editage.cn) for English language editing.
Funding
This project was supported by grants from the National Natural Science Foundation of China (Grant Nos. 81200186, 81670398, and 91639102), Shan-dong Provincial Natural Science Foundation, China (Grant No. ZR2018MC011), Yantai key research and development project (Grant No. 2017WS121), and the funding of Taishan Scholars of Shan-dong Province to Binzhou Medical University.
Author information
Authors and Affiliations
Contributions
Zhen Shi conceived and designed the study; Peng Lu, Li-wen Liang, and Ai-li Xu performed experiments; Zhen Shi, Peng Lu, Li-wen Liang, and Ye-ying Sun analyzed data; Zhen Shi and Shu-jun Jiang wrote the paper; Peng Lu and Li-wen Liang contributed new methods.
Corresponding author
Ethics declarations
All animal work in this study was approved and performed in accordance with the Home Office UK Animals (Scientific Procedures) Act 1986 under the regulations and policies laid out by the Medical Ethics Committee of Binzhou Medical University.
Conflict of interest
The authors declare that they have no conflicts of interest.
Disclaimer
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lu, P., Liang, Lw., Xu, Al. et al. Pro-inflammatory cytokines in the paraventricular nucleus mediate the adipose afferent reflex in rats. Pflugers Arch - Eur J Physiol 472, 343–354 (2020). https://doi.org/10.1007/s00424-020-02356-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02356-7