Abstract
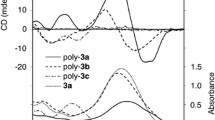
The solution-phase synthesis and cyclisation of three α,β-peptoid octamers with differing side chain patterns is reported. One of these, compound C, showed a significantly greater resolution by NMR relative to the other two structurally related octamers. This observation was studied in detail by circular dichroism at a synchrotron light source to facilitate the correlation between the side chain patterns and conformational preference of these three peptoids. The X-ray crystal structure of cyclic octamer C, the first high-resolution structure for the α,β-peptoid backbone, was also obtained from methanol. Combined solid- and solution-phase studies allowed the identification of the N-2-(benzyloxy)ethyl side chain on the β-residue of the heterogeneous backbone as a key structural feature driving the increased conformational stability for octamer C.








Similar content being viewed by others
Abbreviations
- CD:
-
Circular dichroism
- COSY:
-
Proton–proton correlation experiment
- DMAP:
-
4-Dimethylaminopyridine
- DMF:
-
N,N-Dimethylformamide
- EDC:
-
1-(3-Dimethylaminopropyl)-3-ethyl carbodiimide hydrochloride
- HATU:
-
O-(7-Azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate
- HFIP:
-
1,1,1,3,3,3-Hexafluoroisopropanol
- HMBC:
-
Heteronuclear multiple bond correlation experiment
- HRMS:
-
High-resolution mass spectroscopy
- HSQC:
-
Heteronuclear single quantum correlation experiment
- IR:
-
Infrared
- NMR:
-
Nuclear magnetic resonance
- rt:
-
Room temperature
- SI:
-
Supporting information
- SRCD:
-
Synchrotron radiation circular dichroism
- TFA:
-
Trifluoroacetic acid
- TFE:
-
2,2,2-trifluoroethanol
- THF:
-
Tetrahydrofuran
- TLC:
-
Thin layer chromatography
- UV:
-
Ultraviolet
References
Altomare A, Cascarano G, Giacovazzo C, Guagliardi A (1993) Completion and refinement of crystal structure with SIR92. J Appl Cryst 26:343–350
Armand P, Kirshenbaum K, Falicov A, Dunbrack RL, Dill KA, Zuckermann RN, Cohen FE (1997) Chiral N-substituted glycines can form stable helical conformations. Fold Des 2:369–375
Astle JM, Udugamasooriya DG, Smallshaw JE, Kodadek TA (2008) VEGFR2 Antagonist and other peptoids evade immune recognition. Int J Pept Res Ther 14:223–227
Campbell F, Plante JP, Edwards TA, Warriner SL, Wilson AJ (2010) N-alkylated oligoamide α-helical proteomimetics. Org. Biomol Chem 8:2344–2351
Caumes C, Hjelmgaard T, Remuson R, Faure S, Taillefumier C (2010) Highly convenient gram-scale solution-phase peptoid synthesis and orthogonal side-chain post-modification. Synthesis:257–264
Cecioni S, Faure S, Darbost U, Bonnamour I, Parrot-Lopez H, Roy O, Taillefumier C, Wimmerová M, Praly J-P, Imberty A, Vidal S (2010) Selectivity among two lectins: probing the effect of topology, multivalency and flexibility of “clicked” multivalent glycoclusters. Chem Eur J 17:2146–2159
Clarke DT, Jones G (2004) CD12: a new high flux beamline for ultraviolet and vacuum-ultraviolet circular dichroism on the SRS Daresbury. J. Synchrotron Rad 11:142–149
Combs DJ, Lokey RS (2007) Extended peptoids: a new class of oligomers based on aromatic building blocks. Tetrahedron Lett 48:2679–2682
Flack HD, Schwarzenbach D (1988) On the use of least-square restraint for origin fixing in polar space groups. Acta Cryst A44:499–506
Fowler SA, Blackwell HE (2009) Structure–function relationships in peptoids: recent advances toward deciphering the structural requirements for biological function. Org Biomol Chem 7:1508–1524
Fowler SA, Luechapanichkul R, Blackwell HE (2009) Synthesis and characterization of nitroaromatic peptoids: fine tuning peptoid secondary structure through monomer position and functionality. J Org Chem 74:1440–1449
Gorske BC, Blackwell HE (2006) Tuning peptoid secondary structure with pentafluoroaromatic functionality: a new design paradigm for the construction of discretely folded peptoid structures. J Am Chem Soc 128:14378–14387
Gorske BC, Bastian BL, Geske GD, Blackwell HE (2007) Local and tunable n → pi* interactions regulate amide isomerism in the peptoid backbone. J Am Chem Soc 129:8928–8929
Gorske BC, Stringer JR, Bastian BL, Fowler SA, Blackwell HE (2009) New strategies for the design of folded peptoids revealed by a survey of noncovalent interactions in model systems. J Am Chem Soc 131:16555–16567
Hamper BC, Kolodziej SA, Scates AM, Smith RG, Cortez E (1998) Solid-phase synthesis of β-peptoids: N-Substituted β-aminopropionic acid oligomers. J Org Chem 63:708–718
Hill DJ, Mio MJ, Prince RB, Hughes TS, Moore JS et al (2001) A field guide to foldamers. Chem Rev 101:3893–4012
Hioki H, Kinami H, Yoshida A, Kojima A, Kodama M, Takaoka S, Ueda K, Katsu T (2004) Synthesis of N-substituted cyclic triglycines and their response to metal ions. Tetrahedron Lett 45:1091–1094
Hjelmgaard T, Faure S, Caumes C, De Santis E, Edwards AA, Taillefumier C (2009) Convenient solution-phase synthesis and conformational studies of novel linear and cyclic α, β-alternating peptoids. Org Lett 11:4100–4103
Holub JM, Jang H, Kirshenbaum K (2007) Fit to be tied: conformation-directed macrocyclization of peptoid foldamers. Org Lett 9:3275–3278
Hong DP, Hoshino M, Kuboi R, Goto Y (1999) Clustering of fluorine-substituted alcohols as factor responsible for their marked effect on proteins and peptides. J Am Chem Soc 121:8427–8433
Hu XE, Cassady JM (1995) Selective O-Benzylation of aminoalkanols. Synth Commun 25:907–913
Huang K, Wu CW, Sanborn TJ, Patch JA, Kirshenbaum K, Zuckermann RN, Barron AE, Radhakrishnan I (2006) A Threaded loop conformation adopted by a family of peptoid nonamers. J Am Chem Soc 128:1733–1738
Jávorfi T, Hussain R, Myatt D, Siligardi G (2010) Measuring circular dichroism in a capillary cell using the B23 Synchrotron radiation CD beamline at diamond light source. Chirality 22:E149–E153
Kirshenbaum K, Barron AE, Goldsmith RA, Armand P, Bradley EK, Truong KTV, Dill KA, Cohen FE, Zuckerman RN (1998) Sequence-specific polypeptoids: a diverse family of heteropolymers with stable secondary structure. Proc Nat Acad Sci USA 95:4303–4308
Kwon YU, Kodadek T (2007) Quantitative evaluation of the relative cell permeability of peptoids and peptides. J Am Chem Soc 129:1508–1509
Lee BC, Zuckermann RN, Dill KA (2005) Folding a nonbiological polymer into a compact multihelical structure. J Am Chem Soc 127:10999–11009
Maulucci N, Izzo I, Bifulco G, Aliberti A, De Cola C, Comegna D, Gaeta C, Napolitano A, Pizza C, Tedesco C, Flot D, De Riccardis F (2008) Synthesis, structures, and properties of nine-, twelve-, and eighteen-membered N-benzyloxyethyl cyclic α-peptoids. Chem Commun:3927–3929
Miller SM, Simon RJ, Ng S, Zuckermann RN, Kerr JM, Moos WH (1994) Proteolytic studies of homologous peptide and N-substituted glycine peptoid oligomers. Bioorg Med Chem Lett 4:2657–2662
Moehle K, Hofmann H-J (1996) Peptides and peptoids A quantum chemical structure comparison. Biopolymers 38:781–790
Norgren AS, Zhang SD, Arvidsson PI (2006) Synthesis and circular dichroism spectroscopic investigations of oligomeric beta-peptoids with α-chiral side chains. Org Lett 8:4533–4536
Olsen CA (2010) Peptoid-peptide hybrid backbone architectures. Chembiochem 11:152–160
Olsen CA, Lambert M, Witt M, Franzyk H, Jaroszewski JW (2008) Solid-phase peptide synthesis and circular dichroism study of chiral β-peptoid homooligomers. Amino Acids 34:465–471
Otwinowski Z, Minor W (1997) Processing of X-ray diffraction data collected in oscillation mode. Meth Enzymol 276:307–326
Patch JA, Kirshenbaum K, Seurynck SL, Zuckermann RN, Barron AE (2004) Versatile oligo(N-substituted) glycines: The many roles of peptoids in drug discovery. In: Nielsen PE (ed) Pseudopeptides in Drug Discovery. Wiley-VCH Weinheim, Germany, pp 1–31
Roy O, Faure S, Thery V, Didierjean C, Taillefumier C (2008) Cyclic β-peptoids. Org Lett 10:921–924
Seebach D, Gardiner J (2008) β-peptidic peptidomimetics. Acc Chem Res 41:1366–1375
Seo JW, Barron AE, Zuckermann RN (2010) Novel peptoid building blocks: Synthesis of functionalized aromatic helix-inducing submonomers. Org Lett 12:492–495
Shah NH, Butterfoss GL, Nguyen K, Yoo B, Bonneau R, Rabenstein DL, Kirshenbaum K (2008) Oligo(N-aryl glycines): a new twist on structured peptoids. J Am Chem Soc 130:16622–16632
Sheldrick GM (2008) A short history of SHELX. Acta Cryst A64:112–122
Shin SB, Yoo B, Todaro LJ, Kirshenbaum K (2007) Cyclic peptoids. J Am Chem Soc 129:3218–3225
Simon RJ, Kania RS, Zuckermann RN, Huebner VD, Jewell DA, Banville S, Ng S, Wang L, Rosenberg S, Spellmeyer DC, Tan R, Frankel AD, Santi DV, Cohen FE, Bartlett PA (1992) Peptoids: a modular approach to drug discovery. Proc Natl Acad Sci USA 89:9367–9371
Stringer JR, Crapster JA, Guzei IA, Blackwell HE (2010) Construction of peptoids with all trans-amide backbones and peptoid reverse turns via the tactical incorporation of N-aryl side chains capable of hydrogen bonding. J Org Chem 75:6068–6078
Sui Q, Borchardt D, Rabenstein DL (2007) Kinetics and equilibria of cis/trans isomerization of backbone amide bonds in peptoids. J Am Chem Soc 129:12042–12048
Wien F, Miles AJ, Lees JG, Hoffmann SV, Wallace BA (2005) VUV irradiation effects on proteins in high-flux synchrotron circular dichroism spectroscopy. J Synchrotron Rad 12:517–523
Wu CW, Sanborn TJ, Huang K, Zuckermann RN, Barron AE (2001) Peptoid oligomers with α-chiral, aromatic side chains: sequence requirements for the formation of stable peptoid helices. J Am Chem Soc 123:6778–6784
Wu CW, Kirshenbaum K, Sanborn TJ, Patch JA, Huang K, Dill KA, Zuckermann RN, Barron AE (2003) Structural and spectroscopic studies of peptoid oligomers with α-chiral aliphatic side chains. J Am Chem Soc 125:13525–13530
Yoo B, Kirshenbaum K (2008) Peptoid architectures: elaboration, actuation, and application. Curr Opin Chem Biol 12:714–721
Yoo B, Shin SBY, Huang ML, Kirshenbaum K (2010) Peptoid macrocycles: making the rounds with peptidomimetic oligomers. Chem Eur J 16:5528–5537
Zuckermann RN, Kodadek T (2009) Peptoids as potential therapeutics. Curr Opin Mol Ther 11:299–307
Acknowledgments
We gratefully thank the Carlsberg Foundation for a grant to TH and Aurélie Job and Bertrand Légeret (Clermont-Université, Laboratoire SEESIB) for HPLC and mass spectrometry analysis. AAE gratefully acknowledges the beam time awarded from the Diamond Light Source, UK for the circular dichroism beamline B23. We are greatly indebted to technical team of the “Plateforme de mesures de diffraction X” of the CRM2 (Université de Lorraine).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
De Santis, E., Hjelmgaard, T., Faure, S. et al. Cyclic α,β-peptoid octamers with differing side chain patterns: synthesis and conformational investigation. Amino Acids 41, 663–672 (2011). https://doi.org/10.1007/s00726-011-0887-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-0887-1