Abstract
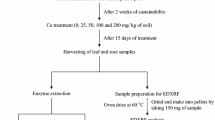
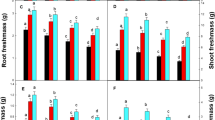
The microlocalisation of Cu was examined in the leaves of white lupin and soybean grown hydroponically in the presence of 1.6 (control) or 192 μM (excess) Cu, along with its effect on leaf morphology, (ultra)structure and the antioxidative response. The 192 μM dose led to a reduction in the total leaf area and leaf thickness in both species, although more strongly so in white lupin. In the latter species it was also associated with smaller spongy parenchyma cells, and smaller spaces between them, while in the soybean it more strongly reduced the size of the palisade parenchyma and epidermal cells. Energy-dispersive X-ray microanalysis showed that under Cu excess the metal was mainly localised inside the spongy parenchyma cells of the white lupin leaves, and in the lower epidermis cell walls in those of the soybean. Cu excess also promoted ultrastructural chloroplast alterations, reducing the photosynthetic capacity index and the green area of the leaves, especially in the soybean. Despite this, soybean appeared to be more tolerant to Cu excess than white lupin, because soybean displayed (1) lower accumulation of Cu in the leaves, (2) enhanced microlocalisation of Cu in the cell walls and (3) greater levels of induced total –SH content and superoxide dismutase and catalase activities that are expected for better antioxidative responses.





Similar content being viewed by others
References
Alaoui-Sossé B, Genet P, Vinit-Dunand F, Toussaint ML, Epron D, Badot PM (2004) Effect of copper on growth in cucumber plants (Cucumis sativus) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci 166:1213–1218
Ali NA, Bernal MP, Ater M (2002) Tolerance and bioaccumulation of copper in Phragmites australis and Zea mays. Plant Soil 239:103–111
Arru L, Rognoni S, Baroncini M, Bonatti PM, Perata P (2004) Copper localization in Cannabis sativa L. grown in a copper-rich solution. Euphytica 140:33–38
Barceló J, Poschenrieder C (2004) Structural and ultrastructural changes in heavy metal exposed plants. In: Prasad MNV, Hagemeyer J (eds) Heavy metal stress in plants. Springer, Heidelberg, pp 223–248
Borghi M, Tognetti R, Monteforti G, Sebastiani L (2008) Responses of two poplar species (Populus alba and Populus × canadensis) to high copper concentrations. Environ Exp Bot 62:290–299
Chamseddine M, Wided BA, Guy H, Marie-Edith C, Fatma J (2009) Cadmium and copper induction of oxidative stress and antioxidative response in tomato (Solanum lycopersicon) leaves. Plant Growth Regul 57:89–99
Chaoui A, El Ferjani E (2005) Effects of cadmium and copper on antioxidant capacities, lignification and auxin degradation in leaves of pea (Pisum sativum L.) seedlings. C R Biol 328:23–31
Chen J, Shiyab S, Han FX, Monts DL, Waggoner CA, Yang Z, Su Y (2009) Bioaccumulation and physiological effects of mercury in Pteris vittata and Nephrolepis exaltata. Ecotoxicol 18:110–121
Cook CM, Vardaka E, Lanaras T (1997) Concentrations of Cu, growth, and chlorophyll content of field-cultivated wheat growing in naturally enriched Cu soil. Bull Environ Contam Toxicol 58:248–253
de Lorenzo C, Fernández-Pascual M, de Felipe MR (1998) Subcellular localization of glycoprotein epitopes during development of lupin root nodules. Protoplasma 201:71–84
Esteban E, Moreno E, Peñalosa JM, Cabrero JI, Millan R, Zornoza P (2008) Short and long-term uptake of Hg in white lupin plants: kinetics and stress indicators. Environ Exp Bot 62:316–322
Fedorova E, Redondo FJ, Koshiba T, de Felipe MR, Pueyo JJ, Lucas MM (2005) Aldehyde oxidase (AO) in the root nodules of Lupinus albus and Medicago truncatula: identification of AO in meristematic and infection zones. Mol Plant Microbe Interact 18:405–413
Fernández-Pascual M, Pueyo JJ, de Felipe MR, Golvano MP, Lucas MM (2007) Singular features of the Bradyrhizobium sp. (Lupinus)–Lupinus symbiosis. Dyn Soil Dyn Plant 1:1–16
Hess FD (1980) Influence of specimen topography on microanalysis. In: Hayat MA (ed) X-ray microanalysis in biology. Macmillan Publishers Ltd, London, pp 241–261
Jones JB Jr (1998) Plant nutrition manual. CRC Press, Boca Raton, pp 1–14
Kasim WA (2005) The correlation between physiological and structural alterations induced by copper and cadmium stress in broad beans (Vicia faba L.). Egypt J Biol 7:20–32
Küpper H, Zhao FJ, McGrath SP (1999) Cellular compartmentation of zinc in leaves of the hyperaccumulator Thlaspi caerulescens. Plant Physiol 119:305–311
Lidon FC, Henriques FS (1993) Changes in the thylakoid membrane polypeptide patterns triggered by excess Cu in rice. Photosynthetica 28:109–117
Lou LQ, Shen ZG, Li XD (2004) The copper tolerance mechanisms of Elsholtzia haichowensis, a plant from copper-enriched soils. Environ Exp Bot 51:111–120
Lowry OH, Roenbrough NJ, Farr AL, Randal EJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lozano-Rodríguez E, Luguera M, Lucena JJ, Carpena-Ruiz RO (1995) Evaluation of two different acid digestion methods in closed systems of trace elements determination in plants. Quim Anal 14:27–30
MacFarlane GR, Burchett MD (2000) Cellular distribution of copper, lead and zinc in the grey mangrove, Avicennia marina (Forsk.). Vierh. Aquat Bot 68:45–59
Maksymiec W, Russa R, Urbanik-Sypniewska T, Baszynski T (1994) Effect of excess Cu on the photosynthetic apparatus of runner bean leaves treated at two different growth stages. Physiol Plant 91:715–721
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Muñoz-Guerra LM (2002) Efecto de la adicción de un residuo orgánico sobre la nutrición mineral de los árboles frutales. Modelización informática de la fertilización y el riego. Tesis Doctoral, Universidad Autónoma de Madrid
Navari-Izzo F, Quartacci MF (2001) Phytoremediation of metals: tolerance mechanisms against oxidative stress. Minera Biotech 13:23–83
Noctor G, Foller CH (1998) Ascorbate and glutathione: keeping active oxygen under control. An Rev Plant Physiol Plant Mol Biol 49:249–279
Panou-Filotheou H, Bosabalidis AM (2004) Root structural aspects associated with copper toxicity in oregano (Origanum vulgare subsp. hirtum). Plant Sci 166:1497–1504
Panou-Filotheou H, Bosabalidis AM, Karataglis S (2001) Effects of Cu toxicity on leaves of oregano (Origanum vulgare subsp. hirtum). Ann Bot 88:207–214
Pätsikkä E, Kairavuo M, Sersen F, Aro EM, Tyystjärvi E (2002) Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol 129:1359–1367
Quartacci MF, Pinzino C, Sgherri CLM, Dalla VF, Navari-Izzo F (2000) Growth in excess copper induces changes in the lipid composition and fluidity of PSII-enriched membranes in wheat. Physiol Plant 108:87–93
Reichman SM (2007) The potential use of the legume-Rhizobium symbiosis for the remediation of arsenic contaminated sites. Soil Biol Biochem 39:2587–2593
Reuter DJ, Robinson JB (1997) Plant analysis: an interpretation manual. 2nd ed. CSIRO Publishing, Melbourne
Romero-Puertas MC, Palma JM, Gómez M, del Rio A, Sandalio LM (2002) Cadmium causes the oxidative modification of proteins in pea plants. Plant Cell Environ 25:677–686
Sánchez-Pardo B, Fernández-Pascual M, Zornoza P (2012) Copper microlocalisation, ultrastructural alterations and antioxidant responses in the nodules of white lupin and soybean plants grown under conditions of copper excess. Environ Exp Bot 84:52–60
Sharma SS, Dietz KJ (2008) The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci 14:43–50
Shi JY, Chen YX, Huang YY, He W (2004) SRXRF microprobe as a technique for studying elements distribution in Elsholtzia splendens. Micron 35:557–564
Srivastava S, Mishra S, Tripathi RD, Dwivedi S, Gupta DK (2006) Copper-induced oxidative stress and responses of antioxidants and phytochelatins in Hydrilla verticillata (L.f.) Royle. Aquat Toxicol 80:405–415
Vajpayee P, Rai UN, Ali MB, Tripathi RD, Kumar A, Singh SN (2005) Possible involvement of oxidative stress in copper-induced inhibition of nitrate reductase activity in Vallisneria spiralis L. Bull Environ Contam Toxicol 74:745–754
Vázquez S, Goldsbrough P, Carpena R (2009) Comparative analysis of the contribution of phytochelatins to cadmium and arsenic tolerance in soybean and white lupin. Plant Physiol Biochem 47:63–67
Yruela I (2009) Copper in plants: acquisition, transport and interactions. Funct Plant Biol 36:409–430
Yurekli F, Porgali ZB (2006) The effects of excessive exposure to copper in bean plants. Acta Biol Cracov Ser Bot 48:7–13
Zornoza P, Vázquez S, Esteban E, Fernández-Pascual M, Carpena R (2002) Cadmium-stress in nodulated white lupin: strategies to avoid toxicity. Plant Physiol Biochem 40:1003–1009
Acknowledgments
Funding for this study was provided by the Spanish MCyT (project CTM2010-21922-C02-02/TECNO), the Autonomous Community of Madrid (project S2009/AMB-1478) and the Junta de Comunidades de Castilla-La Mancha (project POII10-0211-5015). The soybean seeds and Bradyrhizobium japonicum strain were a kind gift of Dr. F. Temprano (IFAPA, Junta de Andalucía). The white lupin seeds were a gift of Dr. A. Gil Aragón (Centro de Investigación Finca La Orden-Valdesequera, Junta de Extremadura). We thank F. Pinto (ICA-CSIC) for expert assistance with LTSEM-EDXMA, S. Fajardo and C. Morcillo (ICA-CSIC) for technical assistance with the microscopy procedures, and Adrian Burton for linguistic assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sánchez-Pardo, B., Fernández-Pascual, M. & Zornoza, P. Copper microlocalisation and changes in leaf morphology, chloroplast ultrastructure and antioxidative response in white lupin and soybean grown in copper excess. J Plant Res 127, 119–129 (2014). https://doi.org/10.1007/s10265-013-0583-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-013-0583-1