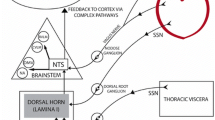
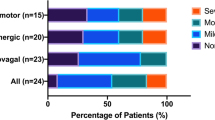
Abstract
The autonomic nervous system (ANS) is the main homeostatic regulatory system of the body. However, this widely distributed neural network can be easily affected by cancer and by the adverse events induced by cancer treatments. In this review, we have classified the ANS complications of cancer into two categories. The first includes direct cancer-related complications, such as primary ANS tumors (pheochromocytoma, paraganglioma or neuroblastoma), as well as autonomic manifestations induced by non-primary ANS tumors (primary brain tumors and metastases). The second comprises indirect ANS complications, which include autonomic features related to cancer therapy (chemotherapy, radiotherapy and/or surgery) and those not related to cancer therapy, such as paraneoplastic autonomic syndromes. We also review the molecular relationship and modulation between the ANS and the cancer cells and their microenvironment.


Similar content being viewed by others
References
Horn JP, Swanson L (2013) The autonomic motor system and the hypothalamus. In: Kandel ER, Schwartz JH, Jesell TM (eds) Principles of neural science, 5th edn. McGraw-Hill Medical, New York, pp 1066–1078
Beissner F, Meissner K, Bär KJ, Napadow V (2013) The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci 33:10503–10511
Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK (2015) Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer 15:563–572
Gidron Y, Perry H, Glennie M (2005) Does the vagus nerve inform the brain about preclinical tumours and modulate them? Lancet Oncol 6:245–248
Bertorini TE, Perez A (2014) Neurologic complications of disorders of the adrenal glands. Handb Clin Neurol 120:749–771
Tischler AS (2008) Pheochromocytoma and extra-adrenal paraganglioma updates. Arch Pathol Lab Med 132:1272–1284
Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, Weiss WA (2016) Neuroblastoma. Nat Rev Dis Primers 2:16078
Pacak K, Linehan WM, Eisenhofer G, Walther MM, Goldstein DSl (2001) Recent advances in genetics, diagnosis, localization, and treatment of pheochromocytoma. Ann Intern Med 134:315–329
Bravo EL (1991) Pheochromocytoma: new concepts and future trends. Kidney Int 40:544–556
Bravo EL, Gifford RW (1984) Current concepts. Pheochromocytoma: diagnosis, localization and management. N Engl J Med 311:1298–1303
Stein PP, Black HRA (1991) A simplified diagnostic approach to pheochromocytoma. A review of the literature and report of one institution’s experience. Medicine (Baltimore) 70:46–66
Jadoul M, Leo JR, Berends MJ, Ooms EC, Buurke EJ, Vasen HF, Seelen PJ, Lips CJ (1989) Pheochromocytoma-induced hypertensive encephalopathy revealing MEN-IIa syndrome in a 13-year old boy. Implications for screening procedures and surgery. Horm Metab Res Suppl 21:46–49
Eclavea A, Gagliardi JA, Jezior J, Burton B, Donahue JP (1997) Phaeochromocytoma with central nervous system manifestations. Australas Radiol 41:373–376
Serter A, Alkan A, Aralasmak A, Kocakoc E (2013) Severe posterior reversible encephalopathy in pheochromocytoma: importance of susceptibility-weighted MRI. Korean J Radiol 14:849–853
Young WF (1993) Pheochromocytoma: 1926–1993. Trends Endocrinol Metab 4:122–127
Kassim TA, Clarke DD, Mai VQ, Clyde PW, Mohamed Shakir KM (2008) Catecholamine-induced cardiomyopathy. Endocr Pract 14:1137–1149
Ruggeri RM, Ferraù F, Campennì A, Simone A, Barresi V, Giuffrè G, Tuccari G, Baldari S, Trimarchi F (2009) Immunohistochemical localization and functional characterization of somatostatin receptor subtypes in a corticotropin releasing hormone-secreting adrenal phaeochromocytoma: review of the literature and report of a case. Eur J Histochem 53:1–6
Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF (2014) Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99:1915–1942
Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Eisenhofer G, King KS, Rao JU, Wesley RA, Adams KT, Pacak K (2012) Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. J Natl Cancer Inst 104:700–708
Grossrubatscher E, Dalino P, Vignati F, Gambacorta M, Pugliese R, Boniardi M, Rossetti O, Marocchi A, Bertuzzi M, Loli P (2006) The role of chromogranin A in the management of patients with phaeochromocytoma. Clin Endocrinol (Oxf) 65:287–293
Ellison DA, Parham DM (2001) Tumors of the autonomic nervous system. Am J Clin Pathol 115(Suppl):S46–S55
Linnoila RI, Keiser HR, Steinberg SM, Lack EE (1990) Histopathology of benign versus malignant sympathoadrenal paragangliomas: clinicopathologic study of 120 cases including unusual histologic features. Hum Pathol 21:1168–1180
Lloyd RV, Sisson JC, Shapiro B, Verhofstad AA (1986) Immunohistochemical localization of epinephrine, norepinephrine, catecholamine-synthesizing enzymes, and chromogranin in neuroendocrine cells and tumors. Am J Pathol 125:45–54
Chen H, Sippel RS, O’Dorisio MS, Vinik AI, Lloyd RV, Pacak K (2010) The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas 39:775–783
Bryant J, Farmer J, Kessler LJ, Townsend RR, Nathanson KL (2003) Pheochromocytoma: the expanding genetic differential diagnosis. J Natl Cancer Inst 95:1196–1204
Burnichon N, Brière JJ, Libé R, Vescovo L, Rivière J, Tissier F, Jouanno E, Jeunemaitre X, Bénit P, Tzagoloff A, Rustin P, Bertherat J, Favier J, Gimenez-Roqueplo AP (2010) SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet 19:3011–3020
Fishbein L, Merrill S, Fraker DL, Cohen DL, Nathanson KL (2013) Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol 20:1444–1450
Unger P, Hoffman K, Pertsemlidis D, Thung S, Wolfe D, Kaneko M (1991) S100 protein-positive sustentacular cells in malignant and locally aggressive adrenal pheochromocytomas. Arch Pathol Lab Med 115:484–487
Chrisoulidou A, Kaltsas G, Ilias I, Grossman AB (2007) The diagnosis and management of malignant phaeochromocytoma and paraganglioma. Endocr Relat Cancer 14:569–585
Vo KT, Matthay KK, Neuhaus J, London WB, Hero B, Ambros PF, Nakagawara A, Miniati D, Wheeler K, Pearson AD, Cohn SL, DuBois SG (2014) Clinical, biologic, and prognostic differences on the basis of primary tumor site in neuroblastoma: a report from the International Neuroblastoma Risk Group Project. J Clin Oncol 32:3169–3176
DuBois SG, Kalika Y, Lukens JN, Brodeur GM, Seeger RC, Atkinson JB, Haase GM, Black CT, Perez C, Shimada H, Gerbing R, Stram DO, Matthay KK (1999) Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol 21:181–189
Hero B, Schleiermacher G (2013) Update on pediatric opsoclonus myoclonus syndrome. Neuropediatrics 44:324–329
Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B (1999) The international neuroblastoma pathology classification (the Shimada system). Cancer 86:364–372
Park JR, Bagatell R, London WB, Maris JM, Cohn SL, Mattay KK, Hogarty M (2013) Children’s Oncology Group’s 2013 blueprint for research: neuroblastoma. Pediatr Blood Cancer 60:985–993
Glantz MJ, Cole BF, Forsyth PA, Recht LD, Wen PY, Chamberlain MC, Grossman SA, Cairncross JG (2000) Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 54:1886–1893
Cayuela N, Simó M, Majós C, Rifà-Ros X, Gállego Pérez-Larraya J, Ripollés P, Vidal N, Miró J, Gil F, Gil-Gil M, Plans G, Graus F, Bruna J (2018) Seizure-susceptible brain regions in glioblastoma: identification of patients at risk. Eur J Neurol 25:387–394
Moseley B, Bateman L, Millichap JJ, Wirrell E, Panayiotopoulos CP (2013) Autonomic epileptic seizures, autonomic effects of seizures, and SUDEP. Epilepsy Behav 26:375–385
Romigi A, Albanese M, Placidi F, Izzi F, Mercuri NB, Marchi A, Liguori C, Campagna N, Duggento A, Canichella A, Ricciardo Rizzo G, Guerrisi M, Marciani MG, Toschi N (2016) Heart rate variability in untreated newly diagnosed temporal lobe epilepsy: evidence for ictal sympathetic dysregulation. Epilepsia 57:418–426
Panayiotopoulos CP (2007) Generalised tonic–clonic seizures. In: Panayiotopoulos CP (ed) A clinical guide to epileptic syndromes and their treatment, 2nd edn. Springer, London, pp 27–38
Panayiotopoulos CP (2007) Symptomatic and cryptogenic (probably symptomatic) focal epilepsies. In: Panayiotopoulos CP (ed) A clinical guide to epileptic syndromes and their treatment, 2nd edn. Springer, London, pp 375–436
Blair RD (2012) Temporal lobe epilepsy semiology. Epilepsy Res Treat 2012:751510
Grisold W, Briani C, Vass A (2013) Malignant cell infiltration in the peripheral nervous system. Handb Clin Neurol 115:685–712
Pancoast HK (1932) Superior pulmonary sulcus tumor: tumor characterized by pain, Horner’s syndrome, destruction of bone and atrophy of hand muscles. JAMA 99:1391–1396
Kori SH, Foley KM, Posner JB (1981) Brachial plexus lesions in patients with cancer: 100 cases. Neurology 31:45–50
Ganju A, Roosen N, Kline DG, Tiel RL (2001) Outcomes in a consecutive series of 111 surgically treated plexus tumours: a review of the experience at the Louisiana State University health sciences center. J Neurosurg 95:51–60
Ferrante MA (2004) Brachial plexopathies: classification, causes, and consequences. Muscle Nerve 30:547–568
Ku A, Lachmann E, Tunkel R, Nagler W (1996) Upper limb reflex sympathetic dystrophy associated with occult malignancy. Arch Phys Med Rehabil 77:726–728
Jaeckle KA, Young DF, Foley KM (1985) The natural history of lumbosacral plexopathy in cancer. Neurology 35:8–15
Jaeckle KA (2004) Neurological manifestations of neoplastic and radiation-induced plexopathies. Semin Neurol 24:385–393
Dalmau J, Graus F, Marco M (1989) ‘Hot and dry foot’ as initial manifestation of neoplastic lumbosacral plexopathy. Neurology 39:871–872
Argyriou AA, Bruna J, Marmiroli P, Cavaletti G (2012) Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol 82:51–77
Krarup-Hansen A, Helweg-Larsen S, Schmalbruch H, Rørth M, Krarup C (2007) Neuronal involvement in cisplatin neuropathy: prospective clinical and neurophysiological studies. Brain 130:1076–1088
Velasco R, Petit J, Clapés V, Verdú E, Navarro X, Bruna J (2010) Neurological monitoring reduces the incidence of bortezomib-induced peripheral neuropathy in multiple myeloma patients. J Peripher Nerv Syst 15:17–25
Haim N, Epelbaum R, Ben-Shahar M, Yarnitsky D, Simri W, Robinson E (1994) Full dose vincristine (without 2-mg dose limit) in the treatment of lymphomas. Cancer 73:2515–2519
Kornblith AB, Anderson J, Cella DF, Tross S, Zuckerman E, Cherin E, Henderson ES, Canellos GP, Kosty MP, Cooper MR (1992) Comparison of psychosocial adaptation and sexual function of survivors of advanced Hodgkin disease treated by MOPP ABVD, or MOPP alternating with ABVD. Cancer 70:2508–2516
Rowinsky EK, McGuire WP, Guarnieri T, Fisherman JS, Christian MC, Donehower RC (1991) Cardiac disturbances during the administration of taxol. J Clin Oncol 9:1704–1712
Meinardi MT, Van Veldhuisen DJ, Gietema JA, Dolsma WV, Boomsma F, Van Den Berg MP, Van Der Graaf WTA (2001) Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol 19:2746–2753
Tjeerdsma G, Meinardi MT, van Der Graaf WT, van Den Berg MP, Mulder NH, Crijns HJ, de Vries EG, van Veldhuisen DJ (1999) Early detection of anthracycline induced cardiotoxicity in asymptomatic patients with normal left ventricular systolic function: autonomic versus echocardiographic variables. Heart 81:419–423
Arab C, Dias DP, Barbosa RT, Carvalho TD, Valenti VE, Crocetta TB, Ferreira M, Abreu LC, Ferreira C (2016) Heart rate variability measure in breast cancer patients and survivors: a systematic review. Psychoneuroendocrinology 68:57–68
Davagnanam I, Fraser CI, Miszkiel K, Daniel CS, Plant GT (2013) Adult Horner’s syndrome: a combined clinical, pharmacological, and imaging algorithm. Eye 27:271–298
Bhandare N, Moiseenko V, Song WY, Morris CG, Bhatti MT, Mendenhall WM (2012) Severe dry eye syndrome after radiotherapy for head-and-neck tumors. Int J Radiat Oncol Biol Phys 82:1501–1508
Pinna R, Campus G, Cumbo E, Mura I, Milia E (2015) Xerostomia induced by radiotherapy: an overview of the physiopathology, clinical evidence, and management of the oral damage. Ther Clin Risk Manag 11:171–188
Magliulo G, Cordeschi S, Sepe C, de Vincentiis M (1998) Taste and lacrimation after acoustic neuroma surgery. Rev Laryngol Otol Rhinol 119:167–170
Prattico F, Perfetti P (2006) Frey’s syndrome. N Engl J Med 355:66
Huang CC, Huang TL, Hsu HC, Chen HC, Lin HC, Chien CY, Fang FM, Chang HW, Tsai NW, Chang WN, Chen SF, Lin TK, Tan TY, Chang CR, Wang HC, Lin WC, Lu CH (2013) Long-term effects of neck irradiation on cardiovascular autonomic function: a study in nasopharyngeal carcinoma patients after radiotherapy. Muscle Nerve 47:344–350
Perino LE, Schuffler MD, Mehta SJ, Everson GT (1986) Radiation-induced intestinal pseudoobstruction. Gastroenterology 91:994–998
Van Duijvendijk P, Slors JF, Taat CW, van Tets WF, van Tienhoven G, Obertop H, Boeckxstaens GE (2002) Prospective evaluation of anorectal function after total mesorectal excision for rectal carcinoma with or without preoperative radiotherapy. Am J Gastroenterol 97:2282–2289
Krol R, Smeenk RJ, van Lin ENJT, Yeoh EEK, Hopman WPM (2014) Systematic review: anal and rectal changes after radiotherapy for prostate cancer. Int J Colorectal Dis 29:273–283
Havenga K, Maas CP, Deruiter MC, Welvaart K, Trimbos JB (2000) Avoiding long-term disturbance to bladder and sexual function in pelvic surgery, particularly with rectal cancer. Semin Surg Oncol 18:235–243
Sharabi Y, Dendi R, Holmes C, Goldstein DS (2003) Baroreflex failure as a late sequela of neck irradiation. Hypertension 42:110–116
Timmers HJ, Karemaker JM, Lenders JW, Wieling W (1999) Baroreflex failure following radiation therapy for nasopharyngeal carcinoma. Clin Auton Res 9:317–324
Shah-Becker S, Pennock M, Sinoway L, Goldenberg D, Goyal N (2017) Baroreceptor reflex failure: review of the literature and the potential impact on patients with head and neck cancer. Head Neck 39:2135–2141
Goyal M, Shukla P, Gupta D, Bisht S, Verma NS, Tiwari S, Bhatt ML (2017) Cardiovascular sequel of neck irradiation in head and neck cancer patients. Int J Radiat Biol 93:711–716
Darnell RB, Posner JB (2011) Paraneoplastic syndromes. In: Darnell RB, Posner JB (eds) Paraneoplastic syndromes, 1st edn. Oxford University Press, Oxford, pp 3–83
Dalmau J, Graus F, Rosenblum MK, Posner JB (1992) Anti-Hu-associated paraneoplastic encephalomyelitis/sensory neuronopathy. A clinical study of 71 patients. Medicine (Baltimore) 71:59–72
Dalmau J, Graus F, Villarejo A, Posner JB, Blumenthal D, Thiessen B, Saiz A, Meneses P, Rosenfeld MR (2004) Clinical analysis of anti-Ma2-associated encephalitis. Brain 127:1831–1844
Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR (2008) Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 7:1091–1098
Graus F, Keime-Guibert F, Reñe R, Benyahia B, Ribalta T, Ascaso C, Escaramis G, Delattre JY (2001) Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain 124:1138–1148
Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J (2000) Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain 123:1481–1494
Hart IK, Maddison P, Newsom-Davis J, Vincent A, Mills KR (2002) Phenotypic variants of autoimmune peripheral nerve hyperexcitability. Brain 125:1887–1895
Höftberger R, Titulaer MJ, Sabater L, Dome B, Rózsás A, Hegedus B, Hoda MA, Laszlo V, Ankersmit HJ, Harms L, Boyero S, de Felipe A, Saiz A, Dalmau J, Graus F (2013) Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology 81:1500–1506
Höftberger R, van Sonderen A, Leypoldt F, Houghton D, Geschwind M, Gelfand J, Paredes M, Sabater L, Saiz A, Titulaer MJ, Graus F, Dalmau J (2015) Encephalitis and AMPA receptor antibodies: novel findings in a case series of 22 patients. Neurology 84:2403–2412
Mason WP, Graus F, Lang B, Honnorat J, Delattre JY, Valldeoriola F, Antoine JC, Rosenblum MK, Rosenfeld MR, Newsom-Davis J, Posner JB, Dalmau J (1997) Small-cell lung cancer, paraneoplastic cerebellar degeneration and the Lambert–Eaton myasthenic syndrome. Brain 120:1279–1300
Petit-Pedrol M, Armangue T, Peng X, Bataller L, Cellucci T, Davis R, McCracken L, Martinez-Hernandez E, Mason WP, Kruer MC, Ritacco DG, Grisold W, Meaney BF, Alcalá C, Sillevis-Smitt P, Titulaer MJ, Balice-Gordon R, Graus F, Dalmau J (2014) Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol 13:276–286
Saiz A, Bruna J, Stourac P, Vigliani MC, Giometto B, Grisold W, Honnorat J, Psimaras D, Voltz R, Graus F (2009) Anti-Hu-associated brainstem encephalitis. J Neurol Neurosurg Psychiatry 80:404–407
Yu Z, Kryzer TJ, Griesmann GE, Kim K, Benarroch EE, Lennon VA (2001) CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol 49:146–154
Vernino S, Sandroni P, Singer W, Low PA (2008) Autonomic ganglia: target and novel therapeutic tool. Neurology 70:1926–1932
Wirtz PW, Smallegange TM, Wintzen AR, Verschuuren JJ (2002) Differences in clinical features between the Lambert–Eaton myasthenic syndrome with and without cancer: an analysis of 227 published cases. Clin Neurol Neurosurg 104(4):359–363
Wabbels BK, Elflein H, Lorenz B, Kolling G (2004) Bilateral tonic pupils with evidence of anti-Hu antibodies as a paraneoplastic manifestation of small cell lung cancer. Ophthalmologica 218:141–143
Lladó A, Mannucci P, Carpentier AF, Paris S, Blanco Y, Saiz A, Delattre JY, Graus F (2004) Value of Hu antibody determinations in the follow-up of paraneoplastic neurologic syndromes. Neurology 63:1947–1949
Walsh D, Nelson KA (2002) Autonomic nervous system dysfunction in advanced cancer. Support Care Cancer 10:523–528
Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR (2008) Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol 26:971–982
Lakoski SG, Jones LW, Krone RJ, Stein PK, Scott JM (2015) Autonomic dysfunction in early breast cancer: incidence, clinical importance, and underlying mechanisms. Am Heart J 170:231–241
Ondicova K, Mravec B (2010) Role of nervous system in cancer aetiopathogenesis. Lancet Oncol 1:596–601
Mancino M, Ametller E, Gascón P, Almendro V (2011) The neuronal influence on tumor progression. Biochim Biophys Acta 1816:105–118
Jobling P, Pundavela J, Oliveira SM, Roselli S, Walker MM, Hondermarck H (2015) Nerve-cancer cell cross-talk: a novel promoter of tumor progression. Cancer Res 1(75):1777–1781
Cole SW, Sood AK (2012) Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res 18:1201–1206
Tang J, Li Z, Lu L, Cho CH (2013) β-Adrenergic system, a backstage manipulator regulating tumour progression and drug target in cancer therapy. Semin Cancer Biol 23:533–542
Lu L, Chen Y, Zhu Y (2017) The molecular basis of targeting PFKFB3 as a therapeutic strategy against cancer. Oncotarget 8:62793–62802
Poulsen H, Morth P, Egebjerg J, Nissen P (2010) Phosphorylation of the Na+ , K+-ATPase and the H+, K+-ATPase. FEBS Lett 584:2589–2595
Romero-Garcia S, Moreno-Altamirano MM, Prado-Garcia H, Sánchez-García FJ (2016) Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front Immunol 7:52
Spindel ER (2012) Muscarinic receptor agonists and antagonists: effects on cancer. Handb Exp Pharmacol 208:451–468
Schuller HM (2009) Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer 9:195–205
Shah N, Khurana S, Cheng K, Raufman JP (2009) Muscarinic receptors and ligands in cancer. Am J Physiol Cell Physiol 296:C221–C232
Campoy FJ, Vidal CJ, Muñoz-Delgado E, Montenegro MF, Cabezas-Herrera J, Nieto-Cerón S (2016) Cholinergic system and cell proliferation. Chem Biol Interact 259:257–265
Zhang XJ, Yang L, Zhao Q, Caen JP, He HY, Jin QH, Guo LH, Alemany M, Zhang LY, Shi YF (2002) Induction of acetylcholinesterase expression during apoptosis in various cell types. Cell Death Differ 9:790–800
Zhao Y (2016) The oncogenic functions of nicotinic acetylcholine receptors. J Oncol 2016:9650481. https://doi.org/10.1155/2016/9650481
Zhang XJ, Greenberg DS (2012) Acetylcholinesterase involvement in apoptosis. Front Mol Neurosci 5:40. https://doi.org/10.3389/fnmol.2012.00040
Sánchez-Osuna M, Yuste VJ (2015) AChE for DNA degradation. Cell Res 25:653–654
Sánchez-Osuna M, Martínez-Escardó L, Granados-Colomina C, Martínez-Soler F, Pascual-Guiral S, Iglesias-Guimarais V, Velasco R, Plans G, Vidal N, Tortosa A, Barcia C, Bruna J, Yuste VJ (2016) An intrinsic DFF40/CAD endonuclease deficiency impairs oligonucleosomal DNA hydrolysis during caspase-dependent cell death: a common trait in human glioblastoma cells. Neuro Oncol 18:950–961
Acknowledgements
This work was partially supported by PI1501303 Grant from Instituto de Salud Carlos III and Fondo Europeo de Desarrollo Regional.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The manuscript does not contain information about not previously published clinical studies or original patient data. The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Simó, M., Navarro, X., Yuste, V.J. et al. Autonomic nervous system and cancer. Clin Auton Res 28, 301–314 (2018). https://doi.org/10.1007/s10286-018-0523-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10286-018-0523-1