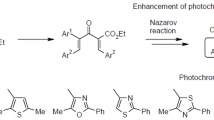
The reaction of tetracyanoethylated 1,2-diarylethanones with morpholine was used for directed synthesis of spiro-fused diarylethenes, 8-amino-1-imino(oxo)-6-morpholino-2-oxa-7-azaspiro[4.4]nona-3,6,8-triene-9-carbonitriles. The intermediates in this process were tetracyanoalkanone salts. The formation of spiranes was sensitive to the nature of aromatic substituents at the carbonyl group of 3,4-diaryl-4-oxobutane-1,1,2,2-tetracarbonitriles. The obtained spiro-fused diarylethenes exhibited photochromic properties.









Similar content being viewed by others
References
Irie, M.; Fukaminato, T.; Matsuda, K.; Kobatake, S. Chem. Rev. 2014, 114, 12174.
Shirinian, V. Z.; Lonshakov, D. V.; Lvov, A. G.; Krayushkin, M. M. Russ. Chem. Rev. 2013, 82, 511. [Usp. Khim. 2013, 82, 511.]
Irie, M. Chem. Rev. 2000, 100, 1685.
Myles, A. J.; Branda, N. R. Adv. Funct. Mater. 2002, 12, 167.
Natali, M.; Giordani, S. Chem. Soc. Rev. 2012, 41, 4010.
Orgiu, E.; Samorì, P. Adv. Mater. 2014, 26, 1827.
Minkin, V. I. Russ. Chem. Bull. 2008, 57, 687. [Izv. Akad. Nauk, Ser. Khim. 2008, 673.]
Szymañski, W.; Beierle, J. M.; Kistemaker, H. A. V.; Velema, W. A.; Feringa, B. L. Chem. Rev. 2013, 113, 6114.
Lonshakov, D. V.; Shirinian, V. Z.; Lvov, A. G.; Krayushkin, M. M. Russ. Chem. Bull. 2012, 61, 1769. [Izv. Akad. Nauk, Ser. Chemistry of Heterocyclic Compounds 2015, 51(6), 518–525 Khim. 2012, 1753.]
Yang, Y.; Xie, Y.; Zhang, Q.; Nakatani, K.; Tian, H.; Zhu, W. Chem.–Eur. J. 2012, 18, 11685.
Liu, G.; Pu, S.; Wang, R. Org. Lett. 2013, 15, 980.
Kitagawa, D.; Sasaki, K.; Kobatake, S. Bull. Chem. Soc. Jpn. 2011, 84, 141.
Takami, S.; Kobatake, S.; Kawai, T.; Irie, M. Chem. Lett. 2003, 32, 892.
Kobatake, S.; Shibata, K.; Uchida, K.; Irie, M. J. Am. Chem. Soc. 2000, 122, 12135.
Nakamura, S.; Yokojima, S.; Uchida, K.; Tsujioka, T.; Goldberg, A.; Murakami, A.; Shinoda, K.; Mikami, M.; Kobayashi, T.; Kobatake, S.; Matsuda, K.; Irie, M. J. Photochem. Photobiol., A 2008, 200, 10.
Yamaguchi, T.; Irie, M. J. Photochem. Photobiol., A 2006, 178, 162.
Kitai, J.; Kobayashi, T.; Uchida, W.; Hatakeyama, M.; Yokojima, S.; Nakamura, S.; Uchida, K. J. Org. Chem. 2012, 77, 3270.
Shirinian, V. Z.; Lvov, A. G.; Krayushkin, M. M.; Lubuzh, E. D.; Nabatov, B. V. J. Org. Chem. 2014, 79, 3440.
Göstl, R.; Kobin, B.; Grubert, L.; Pätzel, M.; Hecht, S. Chem.–Eur. J. 2012, 18, 14282.
Takeshita, M.; Mizukami, E.; Murakami, K.; Wada, Y.; Matsuda, Y. Eur. J. Org. Chem. 2014, 3784.
Jean-Ruel, H.; Gao, M.; Kochman, M. A.; Lu, C.; Liu, L. C.; Cooney, R. R.; Morrison, C. A.; Miller, R. J. D. J. Phys. Chem., B 2013, 117, 15894.
Hanazawa, M.; Sumiya, R.; Horikawa, Y.; Irie, M. J. Chem. Soc., Chem. Commun. 1992, 206.
Celani, P.; Ottani, S.; Olivucci, M.; Bernardi, F.; Robb, M. A. J. Am. Chem. Soc. 1994, 116, 10141.
Jeong, Y.-C.; Park, D. G.; Lee, I. S.; Yang, S. I.; Ahn, K.-H. J. Mater. Chem. 2009, 19, 97.
Kobatake, S.; Uchida, K.; Tsuchida, E.; Irie, M. Chem. Lett. 2000, 1340.
Morimitsu, K.; Shibata, K.; Kobatake, S.; Irie, M. J. Org. Chem. 2002, 67, 4574.
Kitagawa, D.; Sasaki, K.; Kobatake, S. Bull. Chem. Soc. Jpn. 2011, 84, 141.
Kawai, S.; Nakashima, T.; Atsumi, K.; Sakai, T.; Harigai, M.; Imamoto, Y.; Kamikubo, H.; Kataoka, M.; Kawai, T. Chem. Mater. 2007, 19, 3479.
Shirinian, V. Z.; Lvov, A. G.; Yanina, A. M.; Kachala, V. V.; Krayushkin, M. I. Chem. Heterocycl. Compd. 2015, 51, 234. [Khim. Geterotsikl. Soedin. 2015, 51, 234.]
Fukumoto, S.; Nakagawa, T.; Kawai, S.; Nakashima, T.; Kawai, T. Dyes Pigm. 2011, 89, 297.
Morinaka, K.; Ubukata, T.; Yokoyama, Y. Org. Lett. 2009, 11, 3890.
Kühni, J.; Belser, P. Org. Lett. 2007, 9, 1915.
Kobatake, S.; Kuma, S.; Irie, M. J. Phys. Org. Chem. 2007, 20, 960.
Belikov, M. Yu.; Ievlev, M. Yu.; Ershov, O. V.; Lipin, K. V.; Legotin, S. A; Nasakin, O. E. Russ. J. Org. Chem. 2014, 50, 1372. [Zh. Org. Khim. 2014, 50, 1387.]
Belikov, M. Yu.; Ershov, O. V.; Lipovskaya, I. V.; Fedoseev, S. V.; Nasakin, O. E. Russ. J. Org. Chem. 2013, 49, 864. [Zh. Org. Khim. 2013, 49, 880.]
Belikov, M. Yu.; Ershov, O. V.; Lipovskaya, I. V.; Fedoseev, S. V.; Lipin, K. V.; Nasakin O. E. Russ. J. Org. Chem. 2013, 49, 1195. [Zh. Org. Khim. 2013, 49, 1211.].
Carlucci, L.; Ciani, G.; Proserpio, D. M.; Sironi, A. Angew. Chem., Int. Ed. 1996, 35, 1088.
Belikov, M. Yu.; Ershov, O. V.; Eremkin, A. V.; Kayukov, Ya. S.; Nasakin O. E. Russ. J. Org. Chem. 2010, 46, 597. [Zh. Org. Khim. 2010, 46, 604.]
Belikov, M. Yu.; Ershov, O. V.; Eremkin, A. V.; Nasakin, O. E.; Tafeenko, V. A.; Nurieva, E. V. Tetrahedron Lett. 2011, 52, 6407.
Belikov, M. Yu.; Ershov, O. V.; Lipovskaya, I. V.; Eremkin, A. V.; Nasakin, O. E. Russ. J. Org. Chem. 2011, 47, 1426. [Zh. Org. Khim. 2011, 47, 1401.]
Fedoseev, S. V.; Ershov, O. V.; Belikov, M. Yu.; Lipin, K. V.; Bardasov, I. N.; Nasakin, O. E.; Tafeenko, V. A. Tetrahedron Lett. 2013, 54, 2143.
Chen, Y.; Zeng, D. X.; Fan, M. G. Org. Lett. 2003, 5, 1435.
Correia, C.; Carvalho, M. A.; Proença, M. F. Tetrahedron 2009, 65, 6903.
Li, X.; Ma, Y.; Wang, B.; Li, G. Org. Lett. 2008, 10, 3639.
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, A64, 112.
Diamond – Crystal and Molecular Structure Visualization; Release 2.1d; Crystal Impact – Dr. H. Putz & Dr. K. Brandenburg GbR: Bonn, 2000. http://www.crystalimpact.com/diamond
This work received financial support from the Grants Council of the President of Russian Federation (grant MK-97.2014.3).
X-ray structural investigation of salt 3c was performed by using equipment obtained with financial support from the Development Program of the Moscow University and within the framework of Cooperation Agreement between the Department of Chemistry of M. V. Lomonosov Moscow State University and the Chemistry and Pharmacy Department of Chuvashia State University named after I. N. Ulyanov.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2015, 51(6), 518–525
Rights and permissions
About this article
Cite this article
Belikov, M.Y., Ievlev, M.Y., Belikova, I.V. et al. Directed synthesis of new spiro-fused photochromes of diarylethene series. Chem Heterocycl Comp 51, 518–525 (2015). https://doi.org/10.1007/s10593-015-1731-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1731-4