Abstract
Background
The mechanisms for the improvement of the gut flora and the intestinal environment by synbiotic therapy are unclear.
Aims
This study evaluated the changes in the gut flora and the intestinal environment after synbiotic therapy, and tried to clarify the mechanisms by which synbiotic therapy reduces pathological bacteria in the gut.
Methods
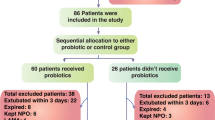
A total of 47 enteral feeding patients with long-term mechanical ventilation support were enrolled in the study. Patients were randomly assigned to synbiotic and control groups, at a two to one ratio. Patients in the synbiotic group were administrated Lactobacillus, Bifidobacterium, and galactooligosaccharides as synbiotics for 8 weeks.
Results
The characteristics of the patients were not significantly different between the control (n = 16) and synbiotic (n = 31) groups. In the synbiotic group, the counts of Bifidobacterium and Lactobacillus in the gut increased significantly to 100 times the initial level following synbiotic treatment. The acetic acid concentration increased (71.1 ± 15.9 vs. 46.8 ± 24.1 μmol/g) and pH decreased in the gut in comparison with the control group. The concentration of acetic acid in the gut increased in proportion to the Bifidobacterium counts. The counts of pathological gram-negative rod decreased significantly to one-tenth of the initial level in inverse proportion to the Bifidobacterium counts. Furthermore, the amount of Pseudomonas aeruginosa in the lower respiratory tract decreased significantly after synbiotic therapy compared to the controls.
Conclusion
Synbiotic therapy reduces the pathological Gram-negative rods by increasing the acetic acid concentration in association with an increased counts of Bifidobacterium.


Similar content being viewed by others
References
Rayes N, Seehofer D, Theruvath T, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation—a randomized, double-blind trial. Am J Transpl. 2005;5:125–130.
Rayes N, Seehofer D, Hansen S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation. 2002;74:123–127.
Rayes N, Hansen S, Seehofer D, et al. Early enteral supply of fiber and Lactobacilli versus conventional nutrition: a controlled trial in patients with major abdominal surgery. Nutrition. 2002;18:609–615.
Kanazawa H, Nagino M, Kamiya S, et al. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch Surg. 2005;390:104–113.
Sugawara G, Nagino M, Nishio H, et al. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg. 2006;244:706–714.
Olah A, Belagyi T, Poto L, Romics L Jr, Bengmark S. Synbiotic control of inflammation and infection in severe acute pancreatitis: a prospective, randomized, double blind study. Hepatogastroenterology. 2007;54:590–594.
Olah A, Belagyi T, Issekutz A, Gamal ME, Bengmark S. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg. 2002;89:1103–1107.
Besselink MG, van Santvoort HC, Buskens E, Boermeester M, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651–659.
Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E. Benefits of a synbiotic formula (Synbiotic 2000Forte) in critically Ill trauma patients: early results of a randomized controlled trial. World J Surg. 2006;30:1848–1855.
Spindler-Vesel A, Bengmark S, Vovk I, Cerovic O, Kompan L. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. J Parenter Enteral Nutr. 2007;31:119–126.
McNaught CE, Woodcock NP, Anderson AD, MacFie J. A prospective randomised trial of probiotics in critically ill patients. Clin Nutr. 2005;24:211–219.
Jain PK, McNaught CE, Anderson AD, Mac Fie J, Mitchell CJ. Influence of synbiotic containing Lactobacillus acidophilus La5, Bifidobacterium lactis Bb 12, Streptococcus thermophilus, Lactobacillus bulgaricus and oligofructose on gut barrier function and sepsis in critically ill patients: a randomised controlled trial. Clin Nutr. 2004;23:467–475.
Shimizu K, Ogura H, Goto M, et al. Synbiotics decrease the incidence of septic complications in patients with severe SIRS: a preliminary report. Dig Dis Sci. 2009;54:1071–1078.
Klarin B, Johansson ML, Molin G, Larsson A, Jeppsson B. Adhesion of the probiotic bacterium Lactobacillus plantarum 299v onto the gut mucosa in critically ill patients: a randomised open trial. Crit Care. 2005;9:R285–R293.
Forestier C, Guelon D, Cluytens V, et al. Oral probiotic and prevention of Pseudomonas aeruginosa infections: a randomized, double-blind, placebo-controlled pilot study in intensive care unit patients. Crit Care. 2008;12:R69.
Knight DJ, Gardiner D, Banks A, et al. Effect of synbiotic therapy on the incidence of ventilator associated pneumonia in critically ill patients: a randomised, double-blind, placebo-controlled trial. Intensive Care Med. 2009;35:854–861.
National Nasocomial infections Surveillance (NNIS). System Report data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485.
Chastere J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903.
Matsuda K, Tsuji H, Asahara T, Kado Y, Nomoto K. Sensitive quantitative detection of commensal bacteria by rRNA-targeted reverse transcription-PCR. Appl Environ Microbiol. 2007;73:32–39.
Matsuda K, Tsuji H, Asahara T, et al. Establishment of an analytical system for the human fecal microbiota, based on reverse transcription-quantitative PCR targeting of multicopy rRNA molecules. Appl Environ Microbiol. 2009;75:1961–1969.
Fujimoto J, Matsuki T, Sasamoto M, Tomii Y, Watanabe K. Identification and quantification of Lactobacillus casei strain Shirota in human feces with strain-specific primers derived from randomly amplified polymorphic DNA. Int J Food Microbiol. 2008;126:210–215.
Kikuchi H, Yajima T. Correlation between water-holding capacity of different types of cellulose in vitro and gastrointestinal retention time in vivo of rats. J Sci Food Agric. 1992;60:139–146.
Matsumoto K, Takada T, Shimizu K, et al. The effects of a probiotic milk product containing Lactobacillus casei strain Shirota on the defecation frequency and the intestinal microflora of sub-optimal health state volunteers: a randomized placebo-controlled cross-over study. Biosci Microflora. 2006;25:39–48.
Matsumoto K, Takada T, Shimizu K, et al. Effects of a probiotic fermented milk beverage containing Lactobacillus casei strain Shirota on defecation frequency, intestinal microbiota, and the intestinal environment of healthy individuals with soft stools. J Biosci Bioeng. 2010;110:547–552.
Amaretti A, Bernardi T, Tamburini E, et al. Kinetics and metabolism of Bifidobacterium adolescentis MB 239 growing on glucose, galactose, lactose, and galactooligosaccharides. Appl Environ Microbiol. 2007;73:3637–3644.
De Vries W, Gerbrandy SJ, Stouthamer AH. Carbohydrate metabolism in Bifidobacterium bifidum. Biochim Biophys Acta. 1967;136:415–425.
Eklund T. The antimicrobial effect of dissociated and undissociated sorbic acid at different pH levels. J Appl Bacteriol. 1983;54:383–389.
Ostling CE, Lindgren SE. Inhibition of enterobacteria and Listeria growth by lactic, acetic and formic acids. J Appl Bacteriol. 1993;75:18–24.
Deibel RH, Niven CF Jr. Pyruvate fermentation by Streptococcus faecalis. J Bacteriol. 1964;88:4–10.
Isakow W, Morrow LE, Kollef MH. Probiotics for preventing and treating nosocomial infections: review of current evidence and recommendations. Chest. 2007;132:286–294.
McNabb B, Isakow W. Probiotics for the prevention of nosocomial pneumonia: current evidence and opinions. Curr Opin Pulm Med. 2008;14:168–175.
Siempos II, Ntaidou TK, Falagas ME. Impact of the administration of probiotics on the incidence of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Crit Care Med. 2010;38:954–962.
Snydman DR. The safety of probiotics. Clin Infect Dis. 2008;46:S104–S111.
Acknowledgments
We wish to express our sincere gratitude for the valuable assistance in performing the bacterial flora analyses by Mr. Norikatsu Yuki and Mr. Akira Takahashi, of the Yakult Central Institute for Microbiological Research. This study was supported by a grant of Hokkaido University.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayakawa, M., Asahara, T., Ishitani, T. et al. Synbiotic Therapy Reduces the Pathological Gram-Negative Rods Caused by an Increased Acetic Acid Concentration in the Gut. Dig Dis Sci 57, 2642–2649 (2012). https://doi.org/10.1007/s10620-012-2201-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2201-9