Abstract
Purpose
To demonstrate an organic (retinal) amblyogenic defect in functional amblyopes not responding to treatment.
Methods
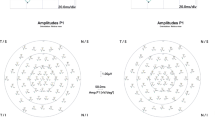
Twenty-four children (Mean age: 5.9 ± 1.8 years; range: 4–10 years) with functional amblyopia were recruited for this study. All these children underwent complete ophthalmic and orthoptic evaluation. In addition, Kinetic Goldman Visual Fields (KGVF), Spectral Domain Optical Coherence Tomography (SD-OCT), full field flash electroretinograms (ffERG) and multifocal electroretinograms (mfERG) were also performed. Ratios were subsequently derived by comparing the amplitudes obtained from the amblyopic eye (AE) to the good eye (GE) for the a- and b-waves of the ffERG, as well as for the ring analysis of the mfERG.
Results
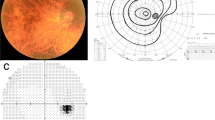
KGVF showed a central scotoma of varying size (3°–7°) and density (absolute to relative), with increasing target size in 14/24 patients whose best post-treatment vision in the AE ranged from 20/100 to 20/40. The scotoma decreased in size and density with improving vision until a plateau of recovery was reached. The remaining 10/24 patients with a vision ≥ 20/30 showed no scotoma. SD-OCT showed no significant difference between the AE and GE. ffERG and mfERG were obtained in 18/24 patients. The ffERG AE/GE ratio was abnormal in 7 patients, 5 of which had large scotomas on KGVF. The mfERG ring 1 AE/GE ratio was significantly (p < .05) attenuated in 9/18 patients out of which 3 were no longer amblyopic. However, there was no significant difference (p > .05) in ring 1 AE/GE amplitude ratio between those who achieved 20/50–20/40 (.81 ± .26) and those with ≥ 20/25(.86 ± .25).
Conclusions
The combined findings of central scotoma on KGVF and mfERG anomalies in patients who did not achieve optimal vision with treatment suggest an underlying organic defect impairing macular function.








Similar content being viewed by others
Data availability
Not applicable.
References
Von Noorden GK (1985) Amblyopia: a multidisciplinary approach. Invest Ophthalmol Vis Sci 26:1704–1716
Beneish R, Williams F, Polomeno RC, Ramsey B, Little JM (1983) Ptosis and amblyopia. Can J Ophthalmol 18:l27-130
Beneish R, Williams F, Polomeno RC, Little JM, Connolly WES (1984) Ultrasonography in unilateral high myopia and amblyopia. In: Ravault AP, Lenk M (eds) Transaction Vth international orthoptic congress. LIPS, Lyon (France), pp 401–413
Beneish R, Williams F, Polomeno RC, Flanders ME (1987) Herpes simplex keratitis and amblyopia. J Pediatr Ophthalmol Strabismus 24:94–96
Beneish R, Lachapelle P, Polomeno RC, Lake N (1990) Pattern VEP differences in strabismic and anisometropic amblyopia. Clin Vision Sci 5:271–283
Lithander J, Sjöstrand J (1991) Anisometropic and strabismic amblyopia in the age group 2 years and above. A prospective study of the results of treatment. Br J Ophthalmol 75:111–116
Levartovsky S, Oliver M, Gottesman N, Shimshoni M (1995) Factors affecting long term results of successfully treated amblyopia: initial acuity and type of amblyopia. Br J Ophthalmol 79:225–228
Flynn JT, Schiffman J, Feuer W, Corona A (1998) The therapy of amblyopia. An analysis of the results of amblyopia therapy utilizing the pooled data of publishes studies. Trans Am Ophthalmol Soc 97:373–395
Ohlsson J, Baumann M, Sjöstrand J, Abrahamson M (2002) Long term visual outcome in amblyopia treatment. Br J Ophthalmol 86:1148–1151
Scott WE, Kutschke PJ, Keech RV, Pfeifer WL, Nichols B, Zhang L (2005) Amblyopia treatment outcomes. J AAPOS 9:107–111
Beneish R, Polomeno RC, Flanders ME, Koenekoop RK (2009) Optimal compliance for amblyopia therapy: occlusion with a translucent tape on the lens. Can J Ophthalmol 44:523–528
Kim SJ, Jeon H, Jung JH, Lee KM, Choi HY (2018) Comparison between over-glasses patching and adhesive patching for children with moderate amblyopia: a prospective randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 256:429–437
Beneish R, Dorfman AL, Khan A, Polomeno RC, Koenekoop RK, Lachapelle P (2015) Electroretinographic evidence supportive of an organic cause in some forms of functional amblyopia. Doc Ophthalmol 130:11–58
Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS, Marmor MF, McCulloch DL, Palmowski-Wolfe AM (2012) International society for clinical electrophysiology of vision. ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc Ophthalmol 124:1–13
McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M (2015) ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130:1–12
Woo J, Jung S, Gauvin M, Lachapelle P (2017) The DTL ERG electrode comes in different shapes and sizes: are they all good? Doc Ophthalmol 135:155–164
Vatcher D, Dorfman AL, Shen Y, You JY, Sun V, Khan A, Polomeno RC, Lachapelle P (2019) Revealing a retinal facilitatory effect with the multifocal ERG. Doc Ophthalmol 138:117–124
Lachapelle P, Benoit J, Little JM, Lachapelle B (1993) Recording the oscillatory potentials of the electroretinogram with the DTL electrode. Doc Ophthalmol 83:119–130
Kurtenbach A, Kramer S, Strasser T, Zrenner E, Langrová H (2017) The importance of electrode position in visual electrophysiology. Doc Ophthalmol 134:129–134
Provis JM, van Driel D, Bilson FA, Russell P (1985) Development of the human retina: patterns of cell distribution and redistribution in the ganglion cell layer. J Comp Neurol 233:429–451
Hendrickson A (1992) A morphological comparison of foveal development in man and monkey. Eye (Lond) 6:136–144
Yuodelis C, Hendrickson A (1986) A qualitative and quantitative analysis of the human fovea during development. Vis Res 26:847–855
Hendrickson A, Drucker D (1992) The development of parafoveal and mid-peripheral human retina. Behav Brain Res 49:21–31
Hendrickson A, Possin D, Vajzovic L, Toth CA (2012) Histologic development of the human fovea from midgestation to maturity. Am J Ophthalmol 154:767–778
Aggrawal DP, Verma G (1980) Static perimetry in the study of amblyopic scotomata. Br J Ophthalmol 64:713–716
Phillipp W, Mayer W (1989) Investigation of visual field defects in strabismic and anisometropic amblyopes with the Octopus program G1. Graefes Arch Clin Exp Ophthalmol 227:448–454
Sireteanu R, Fronius M (1990) Human amblyopia: structure of the visual field. Exp Brain Res 79:603–614
Donahue SP, Wall M, Kutzko KE, Kardon RH (1999) Automated perimetry in amblyopia: a generalized depression. Am J Ophthalmol 127:312–321
Holmes JM, Kraker RT, Beck RW, Birch EE, Cotter SA, Everett DF, Hertle RW, Quinn GE, Repka MX, Scheiman MM, Wallace DK; Pediatric Eye Disease Investigator Group (2003) A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology 110:2075–2087
Greenstein VC, Eggers HM, Hood DC (2008) Multifocal visual evoked potential and automated perimetry abnormalities in strabismic amblyopes. J AAPOS 12:11–17
Barrett BT, Panesar GK, Scally AJ, Pacey IE (2012) Limited role for suppression in the central field of individuals with strabismic amblyopia. PLoS ONE. https://doi.org/10.1371/journal.pone.0036611
Zhang B, Stevenson SS, Cheng H, Laron M, Kumar G, Tong J, Chino YM (2008) Effects of fixation instability on multifocal VEP(mfVEP) responses in amblyopes. J Vis 8(3):1611–1614
Al-Haddad C, Bou Ghannam A, El Moussawi Z, Rachid E, Ismail K, Atallah M, Smeets L, Chahine H (2020) Multifocal electroretinography in amblyopia. Graefes Arch Clin Exp Ophthalmol 258:683–691
Messias A, Reinhard J, Velasco e Cruz AA, Dietz K, MacKeben M, Trauzettel-Klosinski S (2007) Eccentricfixation in stargardt’sdiseaseassessed by Tübingen perimetry. Invest Ophthalmol Vis Sci 48:5815–5822
Huynh SC, Samarawickrama C, Wang XY et al (2009) Macular and nerve fiber layer thickness in amblyopia: the Sydney childhood eye study. Ophthalmology 116:1604–1609
Pang Y, Goodfellow GW, Allison C, Block S, Frantz KA (2011) A prospective study of macular thickness in amblyopic children with unilateral high Myopia. Invest Ophthalmol and Vis Sci 52:2444–2449
Wu SQ, Zhu LW, Xu QB, Xu JL, Zhang Y (2013) Macular and peripapillary retinal nerve fiber layer thickness in children with hyperopic anisometropic amblyopia. Int J Ophthalmol 6:85–89
Yen MY, Cheng CY, Wang AG (2004) Retinal nerve fiber layer thickness in unilateral amblyopia. Invest Ophthalmol and Vis Sci 45:2224–2230
Alotaibi AG, Al Enazi B (2011) Unilateral amblyopia: optical coherence tomography findings. Saudi J Ophthalmol 25:405–409
Repka MX, Kraker RT, Tamkins SM, Suh DW, Nicholas A. Sala NA, Beck RW, for the Pediatric Eye Disease Investigator Group (2009) Retinal nerve fiber layer thickness in amblyopic eyes. Am J Ophthalmol 148:143–147
Esposito Veneruso P, Ziccardi L, Magli G, Parisi V, Falsini B, Magli A (2017) Developmental visual deprivation: long term effects on human cone driven retinal function. Graefes Arch Clin Exp Ophthalmol 255:2481–2486
Praidou A, Hagan R, Newman W, Chandna A (2014) Early diagnosis of stargardt disease with multifocal electroretinogram in children. Int Ophthalmol 34:613–621
Dorfman AL, Vatcher D, Sun V, You JY, Beneish R, Khan A, Lachapelle P (2016) Exploring multifocal electroretinography in pediatrics: a story of patients and patience. Doc Ophthalmol 133:9–41
Baek SC, Ohn YH, Kang SM (2004) Multifocal electroretinograms in amblyopic patients. Invest Ophthalmol and Vis Sci 45:246
Shoushtarian SM, Mirdehghan Farashah MS, Valiollahi P, Tajik A, Adhamimoghaddam F, Malekzadeh S (2010) Electroretinogram in amblyopic and non-amblyopic children. Indian J Pediatr 77:577–578
Wanger P, Persson HE (1984) Oscillatory potentials, flash and pattern-reversal electroretinograms in amblyopia. Acta Ophthalmol (Copenh) 62:643–650
Mendola JD, Conner IP, Roy A, Chan ST, Schwartz TL, Odom JV, Kwong KK (2005) Voxel-based analysis of MRI detects abnormal visual cortex in children and adults with amblyopia. Hum Brain Mapp 25:222–236
Muckli L, Kiess S, Tonhausen N, Singer W, Goebel R, Sireteanu R (2006) Cerebral correlates of impaired grating perception in individual, psychophysically assessed human amblyopes. Vision Res 46:506–526
Li X, Dumoulin SO, Mansouri B, Hess RF (2007) Cortical deficits in human amblyopia: their regional distribution and their relationship to the contrast detection deficit. Invest Ophthalmol Vis Sci 48:1575–1591
Funding
This study was funded by The Research Institute of the McGill University Health Centre, Montreal Children’s Hospital Foundation New Directions in Research Grant (awarded to ALD, RB, AK and PL).
Author information
Authors and Affiliations
Contributions
Raquel Beneish and Allison L. Dorfman equally contributed to this study and should therefore be considered as equal-first authors. RB contributed to study conception and design, orthoptic evaluations, amblyopia treatment, perimetry, data analysis and manuscript preparation. ALD contributed to full field ERG and multifocal ERG recordings and interpretation, data analysis and manuscript preparation. AK contributed to ophthalmic evaluations and manuscript preparation. RCP contributed to ophthalmic evaluations. PL contributed to data analysis and interpretation, manuscript preparation and revision, final approval and submission.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Statements of human rights
All procedures performed on human participants were done so in accordance with the ethical standards of the Institutional Review Board of the McGill University Health Center and in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
All subjects freely consented to participate in this study, and an informed consent was obtained from all participants (or their parents) included in the study.
Statement on the welfare of animals
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Beneish, R., Dorfman, A.L., Khan, A. et al. Organic visual loss measured by kinetic perimetry and retinal electrophysiology in children with functional amblyopia. Doc Ophthalmol 143, 1–16 (2021). https://doi.org/10.1007/s10633-020-09811-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-020-09811-x