Summary
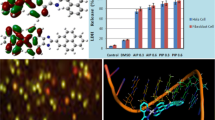
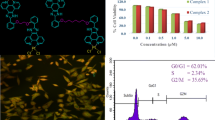
Triphenyltin(IV) complexes of composition [Ph3SnL1H]n (1) and [Ph3SnL2H]n (2) (where L1H = 2-[(E)-2-(3-formyl-4-hydroxyphenyl)-1-diazenyl]benzoate and L2H = 2-[(E)-2-(4-Hydroxy-5-methylphenyl)-1-diazenyl]benzoate) were synthesized and characterized by spectroscopic (1H, 13C and 119Sn NMR, IR, 119Sn Mössbauer) techniques in combination with elemental analysis. The molecular structures and geometries of the complexes (1 and 2) were fully optimized using the quantum mechanical method (PM3). Complexes (1 and 2) were found to exhibit stronger cytotoxic activity in vitro across a panel of human tumour cell lines viz., A498, EVSA-T, H226, IGROV, M19 MEL, MCF-7 and WIDR. The test compounds 1 and 2 exhibit comparable results and both the compounds are found to be far superior to CCDP (cisplatin), 5-FU (5-fluorouracil) and ETO (etoposide) across a panel of cell lines and the activity is more pronounced for the A498 (22 fold) and H226 (33 fold) cell lines compared to CCDP, and A498 (13 fold), H226 (39 fold) and MCF-7 (33 fold) cell lines compared to ETO. The test compounds are even 23 fold more active in magnitude in terms of the ID50 value at least against the H226 cell lines when compared with MTX (methotrexate). Further, the mechanistic role of cytotoxic activity of test compounds (1 and 2), are discussed in relations to the theoretical results of docking studies with some of the key enzymes such as ribonucleotide reductase, thymidylate synthase, thymidylate phosphorylase and topoisomerase II.










Similar content being viewed by others
References
Shuaibu MN, Kanbara H, Yanagi T, Ichinose A, Ameh DA, Bonire JJ, Nok AJ (2003) In vitro trypanocidal activity of dibutyltin dichloride and its fatty acid derivatives. Jpn Parasitolog Res 91:5–11
Jan C-R, Jiann B-P, Lu Y-C, Chang H-T, Su W, Chen W-c YuC-C, Huang J-K (2002) Effect of the organotin compound triethyltin on Ca2+ handling in human prostate cancer cells. Life Sci 70:1337–1345
Samuel MP, de Vos D, Raveendra D, Sarma JARP, Roy S (2002) 3-D QSAR studies on new dibenzyltin(IV) anticancer agents by comparative molecular field analysis (CoMFA). Bioorg Med Chem Lett 12:61–64
Carraher CE Jr, Battin A, Shahi KR, Roner MR (2007) Synthesis, structural characterization, and initial evaluation as anticancer Drugs of dibutyltin polyamines derived from various 4, 6-diaminopyrimidines. J Inorg Organomet Polym 17:631–639
Barot G, Shahi KR, Roner MR, Carraher CE Jr (2007) Synthesis, structural characterization, and ability to inhibit cancer growth of a series of organotin poly(ethylene glycols). J Inorg Organomet Polym 17:595–603
Roner M, Carraher C, Sabir T, Shahi K, Roehr J, Bassett K (2006) Anticancer and antiviral activities of organotin polyether amines derived from the antiviral acyclovir. Polym Mater Sci Eng 95:525–527
Carraher C, Ashida Y, Battin G (2006) Synthesis of organotin polyethers containing diethylstilbestrol. Polym Mater Sci Eng 95:556–558
Carraher C, Sabir T, Roner M, Shahi K, Bleicher R, Roehr J, Bassett K (2006) Synthesis of organotin polyamine ethers containing acyclovir and their preliminary anticancer and antiviral activity. J Inorg Organomet Polym 16:249–257
Roner M, Carraher C, Roehr J, Bassett K (2006) Antiviral and anticancer activity of organotin polymers and reactants derived from norfloxacin and ampicillin. J Polym Mater 23:153–159
Carraher C, Siegmann-Louda D (2004) Macromolecles containing metal and metal-like elements, vol 3. Biomedical applications. Wiley, Hoboken
Doucette R, Siegmann-Louda D, Carraher C, Cardoso A (2004) Inhibition of Balb 3T3 cells as a function of metal for kinetin containing polymers. Polym Mater Sci Eng 91:564–566
Blower PJ (2004) Inorganic pharmaceuticals. Annu Rep Prog Chem Sect A 100:633–658
Blunden SJ, Evans CJ (1990) In: Hutziger O (ed.) Anthropogenic compounds. Springer, Heidelberg, pp 1–44
Evans CJ (1998) In: Smith PJ (ed.) Chemistry of tin. Blackie Academic, London, pp 442–479
Willem R, Bouhdid A, Mahieu B, Ghys L, Biesemans M, Tiekink ERT, de Vos D, Gielen M (1997) Synthesis, characterization and in vitro antitumour activity of triphenyl- and tri-n-butyltin benzoates, phenylacetates and cinnamates. J Organomet Chem 531:151–158
Gielen M, El Khloufi A, Biesemans M, Bouhdid A, de Vos D, Mahieu B, Willem R (1994) Synthesis, characterization and high in vitro antitumour activity of novel triphenyltin carboxylates. Met-Based Drugs 1:305–309
Bouălam M, Gielen M, El Khloufi A, de Vos D, Willem R, Novel (1993) organo-tin compounds having anti-tumour activity and anti-tumour compositions, Pharmachemie B.V., Eur Pat , Publ 538 517, Appl. 91/202, 746.3-, 22.10.91; Chem Abstr 119, 117548b
Gielen M, Willem R, Biesemans M, Bouălam M, El Khloufi A, de Vos D (1992) Exceptionally high in vitro antitumour activity of substituted triphenyltin benzoates including salicylates against a human mammary tumour, MCF-7, and a colon carcinoma, WiDr. Appl Organomet Chem 6:287–291
Gielen M, Tiekink ERT (eds) (2005) Metallotherapeutic drug and metal-based diagnostic agents: 50Sn Tin compounds and their therapeutic potential. Wiley, Chichester, England, pp 421–439 (and references therein)
Kemmer M, Gielen M, Biesemans M, de Vos D, Willem R (1998) Synthesis, characterization and in vitro antitumour activity of di-n-butyl, tri-n-butyl and triphenyltin 3, 6-dioxaheptanoates and 3, 6, 9-trioxadecanoates. Met-Based Drugs 5:189–196
Kemmer M, Ghys L, Gielen M, Biesemans M, Tiekink ERT, Willem R (1999) Synthesis and characterization of triphenyl-, tri-n-butyl and di-n-butyltin derivatives of 4-carboxybenzo-18-crown-6 and -15-crown-5. J Organomet Chem 582:195–203
Gielen M, Lelieveld P, de Vos D, Pan H, Willem R, Biesemans M, Fiebig HH (1992) In vitro effect of organotin-substituted steroids in human tumor cell lines. Inorg Chim Acta 196:115–117
Gielen M, Willem R, Dalil H, de Vos D, Kuiper CM, Peters GJ (1998) Toxicity profiles in vivo in mice and antitumour activity in tumour-bearing mice of di- and triorganotin compounds. Met-Based Drugs 5:83–90
Dakternieks D, Basu Baul TS, Dutta S, Tiekink ERT (1998) Synthesis, characterization, and X-ray structures of diphenyltin(IV) N-(2-hydroxyacetophenone)glycinate, its 1:1 adduct with triphenyltin(IV) chloride, and related systems. Organometallics 17:3058–3062
Basu Baul TS, Dutta S, Tiekink ERT (1999) Crystal structure of diphenyltin(IV) N-(2-hydroxy-5-methylacetophenone)glycinate, C23H21NO3Sn. Z Kristallogr (NCS) 214:361–362
Basu Baul TS, Dutta S, Rivarola E, Choudhuri S (2001) Synthesis, characterization of diorganotin(IV) complexes of N-(2-hydroxyarylidine)aminoacetic acid and antitumour screening in vivo in Ehrlich ascites carcinoma cells. Appl Organomet Chem 15:947–953
Basu Baul TS, Dutta S, Rivarola E, Butcher R, Smith FE (2002) The synthesis and structural characterization of some triorganotin(IV) complexes of 2-{[(E)-1-(2-hydroxyaryl)alkylidene]amino}acetic acid. Crystal and molecular structures of Ph3Sn(2-OHC6H4C(H)=NCH2COO) and Me3Sn(2-OHC6H4C(CH3)=NCH2COO). J Organomet Chem 654:100–108
Basu Baul TS, Dutta S, Masharing C, Rivarola E, Englert U (2003) Organotin(IV) complexes of N-[(2Z)-3-hydroxy-1-methyl-2-butenylidene]glycine. Heteroatom Chem 14:149–154
Yin H, Wang Q, Xue S (2004) Synthesis and structural characterization of diorganotin(IV) esters of salicylidene-amino acids. J Organomet Chem 689:2480–2485
Basu Baul TS, Masharing C, Willem R, Bieseman M, Holčapek M, Jirásko R, Linden A (2005) Self-assembly of diorganotin(IV) 2-{[(E)-1-(2-oxyaryl)alkylidene]amino}acetates: an investigation of structures by X-ray diffraction, solution and solid state tin NMR, and electrospray ionisation MS. J Organomet Chem 690:3080–3094
Linden A, Basu Baul TS, Masharing C (2005) Chloro{μ-2-[(E)-1-(2-oxido-3-methylphenyl)- ethylideneamino]acetato}pentaphenylditin(IV). Acta Crystallogr E61:m557–m559
Basu Baul TS, Masharing C, Basu S, Rivarola E, Holčapek M, Jirásko R, Lyčka A, de Vos D, Linden A (2006) Synthesis, characterization, cytotoxic activity and crystal structures of tri- and di-organotin(IV) complexes constructed from the β-{[(E)-1-(2-hydroxyaryl)alkylidene]amino}propionate and β-{[(2Z)-(3-hydroxy-1-methyl-2-butenylidene)]amino}propionate skeletons. J Organomet Chem 691:952–965
Basu Baul TS, Masharing C, Rivarola E, Smith FE, Butcher J (2006) Synthesis and characterization of tribenzyltin(IV) and dibenzyltin(IV) complexes of 2-{[(2Z)-3-hydroxy-1-methyl-2-butenylidene]amino}acetic acid. Crystal structure of tribenzyl{2-{[(2Z)-3-hydroxy-1-methyl-2-butenylidene]amino}acetate}tin(IV). Struct Chem 18:231–235
Basu Baul TS, Masharing C, Ruisi G, Jirásko R, Holčapek M, de Vos D, Wolstenholme D, Linden A (2007) Self-assembly of extended schiff base amino acetate skeletons, 2-{[(2Z)-(3-hydroxy-1-methyl-2-butenylidene)]amino}phenylpropionate and 2-{[(E)-1-(2-hydroxyaryl)alkylidene]amino}phenylpropionate skeletons incorporating organotin(IV) moieties: synthesis, spectroscopic characterization, crystal structures, and in vitro cytotoxic activity. J Organomet Chem 692:4849–4862
Basu Baul TS, Basu S, de Vos D, Linden A (2009) Amino acetate functionalized Schiff base organotin(IV) complexes as anticancer drugs: synthesis, structural characterization, and in vitro cytotoxicity studies. Invest New Drugs . doi:10.1007-s10637-008-9189-1
Basu Baul TS, Rynjah W, Willem R, Biesemans M, Verbruggen I, Holčapek M, de Vos D, Linden A (2004) Dibutyltin(IV) complexes of the 5-[(E)-2-(Aryl)-1-diazenyl]-2-hydroxybenzoic acid ligand: an investigation of structures by X-ray diffraction, solution and solid state tin NMR, electrospray ionization MS and assessment of in vitro cytotoxicity. J Organomet Chem 689:4691–4701
Basu Baul TS, Rynjah W, Rivarola E, Lyčka A, Holčapek M, Jirásko R, de Vos D, Butcher RJ, Linden A (2006) Synthesis and characterization of bis[dicarboxylatotetraorganodistannoxane] units involving 5-[(E)-2-(Aryl)-1-diazenyl]-2-hydroxybenzoic acids: an investigation of structures by X-ray diffraction, NMR, electrospray ionization MS and assessment of in vitro cytotoxicity. J Organomet Chem 691:4850–4862
Singh P, Bhardwaj A (2008) Mechanism of action of key enzymes associated with cancer propagation and their inhibition by various chemotherapeutic agents. Mini Rev Med Chem 8:388–398
Kushlefsky B, Simmons I, Ross A (1963) Characterization of triphenyltin hydroxide and bis-(triphenyltin) oxide. Inorg Chem 2:187–189
Basu Baul TS, Pyke SM, Sarma KK, Tiekink ERTT (1996) Crystal and molecular structure of aquatriphenyltin 2-(3-formyl-4-hydroxyphenylazo)benzoate. Main Group Met Chem 19:807–814
Boyd MR (1989) Status of the NCI preclinical antitumor drug discovery screen. Principles and practice of oncology, vol 3, pp 1–12. Lippincott, Philadelphia
Keepers YP, Pizao PR, Peters GJ, Ark-Otte JV, Winograd B, Pinedo HM (1991) Comparison of the sulforhodamine B protein and tetrazolium (MTT) assays for in vitro chemosensitivity testing. Eur J Cancer 27:897–900
Stewart JJP (1989) Optimization of parameters for semiempirical methods: I. method. J Comput Chem 10:209–220
Stewart JJP (1989) Optimization of parameters for semiempirical methods: II. applications. J Comput Chem 10:221–264
Stewart JJP (1991) Optimization of parameters for semiempirical methods: III. Extension of PM3 to Be, Mg, Zn, Ga, Ge, As, Se, Cd, In, Sn, Sb, Te, Hg, Tl, Pb, and Bi. J Comput Chem 12:320–341
Stewart JJP (2004) Optimization of parameters for semiempirical methods IV. Extension of MNDO, AM1, and PM3 to more main group elements. J Mol Model 10:155–164
Arguslab 4.0.1: Thompson MA (2004) Planaria Software LLC, Seattle, WA, http://www.argusLab.com
Protein Data Bank, <http://www.rcsb.org/pdb/>
Wang R, Lai L, Wang S (2002) Further development and validation of empirical scoring functions for structure-based binding affinity prediction. J Comput Aided Mol Des 16:11–26
Holeček J, Nádvorník M, Handliř K, Lyčka A (1983) 13C and 119Sn NMR Study of some four- and five-coordinate triphenyltin(IV) compounds. J Organomet Chem 241:177–184
Willem R, Verbruggen I, Gielen M, Biesemans M, Mahieu B, Basu Baul TS, Tiekink ERT (1998) Correlating Mössbauer, solution and solid state 117Sn NMR data with X-ray diffraction structural data of triorganotin 2-[(E)-2-(2-hydroxy-5-methylphenyl)-1-diazenyl]benzoates. Organometallics 17:5758–5766
Barbieri R, Huber F, Pellerito L, Ruisi G, Silvestri A (1998) In: Smith PJ (ed), Chemistry of tin: 119Sn Mössbauer studies on tin compounds. Blackie, London, pp 496–540
Corral E, Hotze ACG, den Dulk H, Leczkowska A, Rodger A, Hannon MJ, Reedijk J (2009) Ruthenium polypyridyl complexes and their modes of interaction with DNA: is there a correlation between these interactions and the antitumor activity of the compounds? J Biol Inorg Chem 14:439–448
Nath M, Pokharia S, Song X, Eng G, Gielen M, Kemmer M, Biesemans M, Willem R, de Vos D (2003) New organotin(IV) derivatives of dipeptides as models for metal-protein interactions: in vitro anti-tumour activity. Appl Organomet Chem 17:305–314
Roberts JJ, Pascoe JM (1972) Cross-linking of complementary strands of DNA in mammalian cells by antitumour platinum compounds. Nature 235:282–284
Williams DR (1974) Bioinorganic Drugs. Part 1. Educ Chem 11:124–127
Saxena AK, Huber F (1989) Organotin compounds and cancer chemotherapy. Coord Chem Rev 95:109–123
Acknowledgements
The financial support of the Department of Science & Technology, New Delhi, India (Grant No.SR/S1/IC-03/2005,TSBB and SR/S1/OC-11A/2006, PS), of the Università degli Studi di Palermo, Italy (Grants ORPA079E5M and ORPA0737W2) and the University Grants Commission, New Delhi, India through SAP-DSA, Phase-III, are gratefully acknowledged. The in vitro cytotoxicity experiments were carried out by Ms. P. F. van Cuijk in the Laboratory of Translational Pharmacology, Department of Medical Oncology, Erasmus Medical Center, Rotterdam, The Netherlands, under the supervision of Dr. E. A. C. Wiemer and Prof. Dr. G. Stoter.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tushar S. Basu Baul dedicates this paper to his collaborator Dr. D. de Vos on his retirement, who was a key person in his research career.
Rights and permissions
About this article
Cite this article
Basu Baul, T.S., Paul, A., Pellerito, L. et al. Triphenyltin(IV) 2-[(E)-2-(aryl)-1-diazenyl]benzoates as anticancer drugs: synthesis, structural characterization, in vitro cytotoxicity and study of its influence towards the mechanistic role of some key enzymes. Invest New Drugs 28, 587–599 (2010). https://doi.org/10.1007/s10637-009-9293-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-009-9293-x