Abstract
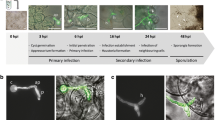
We analyzed the infection of the model plant Arabidopsis thaliana by the basidiomycete phytopathogen of cereals Sporisorium reilianum in order to use it as an experimental pathosystem model. Sterile plantlets of A. thaliana were grown on MS solid medium, and inoculated with haploid strains or mixtures of sexually compatible S. reilianum strains. Inoculated plants showed growth of filaments within their tissues, size reduction, a drastic increase in the formation of lateral roots, and a high production of plant pigments. Although symptoms were more severe in plants infected with sexually compatible strains, no spores were formed by the fungus. Among the pigments accumulated in the stunted plants we identified the anthocyanins cyanidin, cyanidin 3-glucoside, malvidin and pelargonidin. Congruently, the anthocyanin biosynthesis genes: chalcone synthase (CHS, AT5G13930) and dihydroflavonol reductase (DFR, AT5G42800) were over-expressed. The three genes encoding flavin monooxygenases: YUCCA7 (YUC7, AT2G33230), YUCCA8 (YUC8, AT4G28720), and YUCCA9 (YUC9, AT1G04180), and the transcription factor Jagged Lateral Organs (JLO, AT4G00220), all of them involved in the biosynthesis of auxins specific for root development, were also positively regulated. These data provide evidence that both haploid and the mixture of sexually compatible strains of S. reilianum can infect plants of A. thaliana; evidencing the usefulness of this pathosystem for the study of the genetic and metabolic reactions involved in S. reilianum virulence.








Similar content being viewed by others
References
Abdel-Aal, E. S. M., & Hucl, P. (1999). A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chemistry, 76, 350–354.
Banuett, F. (1995). Genetics of Ustilago maydis, a fungal pathogen that induces tumors in maize. Annual Review of Genetics, 29, 179–208.
Banuett, F., & Herskowitz, I. (1989). Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proceedings of the National Academy of Sciences of the United States of America, 86, 5878–5882.
Belhaj, K., Cano, L. M., Prince, D. C., Kemen, A., Yoshida, K., Dagdas, Y. F., Etherington, G. J., Schoonbeek, H. J., van Esse, H. P., Jones, J. D. G., Kamoun, S., & Schornack, S. (2017). Arabidopsis late blight: infection of a nonhost plant by Albugo laibachii enables full colonizationby Phytophthora infestans. Cellular Microbiology, 19, e12628. https://doi.org/10.1111/cmi.12628.
Bhaskaran, S., & Smith, R. H. (1993). Carbohydrates, inertase activity, growth and dimorphism in Sporisorium reilianum. Mycopathologia, 122, 35–41.
Cai, X. T., Xu, P., Zhao, P. X., Liu, R., Yu, L. H., & Xiang, C. B. (2014). Arabidopsis ERF109 mediates cross-talk between jasmonic acid and auxin biosynthesis during lateral root formation. Nature Communications, 5, 5833. https://doi.org/10.1038/ncomms6833.
Davis, K. R. (1993). Arabidopsis thaliana as a model for plant-pathogen interactions. In R. Hammershmidt (Ed.), The American phytopathological society (pp. 1–134). APS Press: St. Paul.
Doehlemann G., Ökmen B., Zhu W., & Sharon A. (2017). Plant pathogenic fungi. Microbiology Spectrum, 5. https://doi.org/10.1128/microbiolspec.FUNK-0023-2016.
Garzón, G. A. (2008). Anthocyanins as natural colorants and bioactive compounds. A review. Acta Biológica Colombiana, 13, 27–36.
Hoch, H. C., Galvani, C. D., Szarowski, D. H., & Turner, J. N. (2005). Two new fluorescent dyes applicable for visualization of fungal cell walls. Mycologia, 97, 580–588.
Holliday, R. (1974). Ustilago maydis. In R. C. King (Ed.), The handbook of genetics (pp. 575–595). New York: Plenum Press.
Ibraheem, F., Gaffoo, I., Tan, Q., Shyu, C. R., & Chopra, S. (2015). A sorghum MYB transcription factor induces 3-deoxyanthocyanidins and enhances resistance against leaf blights in maize. Molecules, 20, 2388–2404.
Khoo, H. E., Azlan, A., Tang, S. T., & Lim, S. M. (2017). Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food & Nutrition Research, 61, 1361779.
Koes, R., Verweij, W., & Quattrocchio, F. (2005). Flavonoids: a colorful model for the regulation and evolution of biotechemical pathways. Trends in Plant Science, 10, 236–242.
Kondo, S., Hiraoka, K., Kobayashi, S., Honda, C., & Terahara, N. (2002). Changes in the expression of anthocyanin biosynthetic genes during apple development. Journal of the American Society for Horticultural Science, 127, 971–976.
Kontoyiannis, D. P., & Lewis, R. E. (2010). Studying fungal pathogenesis in Drosophila. Microbe, 5, 291–295.
Li, N., Wu, H., Ding, Q., Li, H., Li, Z., Ding, J., & Li, Y. (2018). The heterologous expression of Arabidopsis PAP2 induces anthocyanin accumulation and inhibits plant growth in tomato. Functional & Integrative Genomics, 18, 341–353. https://doi.org/10.1007/s10142-018-0590-3.
Martin, F., Delareulle, C., & Hilbert, J. L. (1990). An improved ergosterol assay to estimate fungal biomass in ectomycorrhizas. Mycological Research, 94, 1059–1064.
Martinez, C., Roux, C., Jauneau, A., & Dargent, R. (2002). The biological cycle of Sporisorium reilianum f. sp. zeae: an overview using microscopy. Mycologia, 94, 505–514.
Martínez-Soto, D., Robledo-Briones, A. M., Estrada-Luna, A. A., & Ruiz-Herrera, J. (2013). Transcriptomic analysis of Ustilago maydis infecting Arabidopsis reveals important aspects of the fungus pathogenic mechanisms. Plant Signaling & Behavior. https://doi.org/10.4161/psb.25059.
Martínez-Soto, D., Pérez-Garcia, F. E., & Ruiz-Herrera, J. (2016). Arabidopsis infection by haploid or diploid strains of Ustilago maydis reveals its capacity as a necrophic or biotrophic phytopathogen. Fungal Genomics and Biology. https://doi.org/10.4172/2165-8056.1000133.
Mazaheri-Naeini, M., Sabbagh, S. K., Martinez, Y., Séjalon-Delmas, N., & Roux, C. (2015). Assessment of Ustilago maydis as a fungal model for root infection studies. Fungal Biology, 119, 145–153.
Méndez-Morán, L., Reynaga-Peña, C. G., Springer, P. S., & Ruiz-Herrera, J. (2005). Ustilago maydis infection of the nonnatural host Arabidopsis thaliana. Phytopathology, 95, 480–488.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15, 473–497.
Olatunji, D., Geelen, D., & Verstraeten, I. (2017). Control of endogenous auxin levels in plant root development. International Journal of Molecular Sciences. https://doi.org/10.3390/ijms18122587.
Palomeros-Suárez, P. A., Massange-Sánchez, J. A., Sánchez-Segura, L., et al. (2017). AhDGR2, an amaranth abiotic stress-induced DUF642 protein gene, modifies cell wall structure and composition and causes salt and ABA hyper-sensibility in transgenic Arabidopsis. Planta, 245, 623–640.
Perfect, J. R., Savani, D. V., & Durack, D. T. (1986). Comparison of itraconazole and fluconazole in treatment of cryptococcal meningitis and candida pyelonephritis in rabbits. Antimicrobial Agents and Chemotherapy, 29, 579–583.
Poloni, A., & Schirawski, J. (2016). Host specificity in Sporisorium reilianum is determined by distinct mechanisms in maize and sorghum. Molecular Plant Pathology, 17, 741–754.
Rast-Somssich, M. I., Zádníkova, P., Schmid, S., Kieffer, M., Kepinski, S., & Simon, R. (2017). The Arabidopsis Jagged Lateral Organs (JLO) gene sensitizes plants to auxin. Journal of Experimental Botany, 68, 2741–2755.
Schirawski, J., Heinze, B., Wagenknecht, M., & Kahmann, R. (2005). Mating type loci of Sporisorium reilianum: novel pattern with three a and multiple b specificities. Eukaryotic Cell, 4, 1317–1327.
Schirawski, J., Mannhaupt, G., Münch, K., et al. (2010). Pathogenicity determinants in smut fungi revealed by genome comparison. Science, 330, 1546–1548.
van der Does, H. C., & Rep, M. (2017). Adaptation to the host environment by plant-pathogenic fungi. Annual Review of Phytopathology, 55, 427–450.
Warpeha, K. M., Park, Y. D., & Williamson, P. R. (2013). Susceptibility of intact germinating Arabidopsis thaliana to human fungal pathogens Cryptococcus neoformans and C. gattii. Applied and Environmental Microbiology, 79, 2979–2988.
Weigel, D., & Glazebrook, J. (2002). Arabidopsis: A laboratory manual. New Yok: Cold Spring Harbor Laboratory Press.
Zhao, Y. (2012). Auxin biosynthesis: a simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Molecular Plant, 5, 334–338.
Zuther, K., Kahnt, J., Utermark, J., Imkampe, J., Uhse, S., & Schirawski, J. (2012). Host specificity of Sporisorium reilianum is tightly linked to generation of the phytoalexin luteolinidin by Sorghum bicolor. Molecular Plant-Microbe Interactions, 25, 1230–1237.
Acknowledgments
This work was partially supported by Consejo Nacional de Ciencia y Tecnología (Conacyt), México. Thanks are given to Prof. Jan Schirawski, Institute of Applied Microbiology, RWTH Aachen University, Germany, for making available the S. reilianum strains SRZ2 and SRZ1. We also thank Mayela F. Salazar-Chávez from the Irapuato Unit of Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional (Cinvestav), Leandro A. Núñez-Muñoz and Brenda J. Vargas-Hernández from Zacatenco Unit of Cinvestav; Renata Cruz-Calderon from Instituto Tecnológico Superior de Los Reyes, TSLR; and Drs. José I. Reyes-Olalde, and Stefan de Folter from the Unit of Advance Genomics, Cinvestav, for their assistance respectively in strain maintenance, preparation of some reagents, statistical analyses, and microscopic analyses.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they are aware of the ethical responsibilities required by the European Journal of Plant Pathology for the submission of manuscripts. Also, they declare no conflict of interests and that they were informed of the submission of the manuscript.
Rights and permissions
About this article
Cite this article
Martínez-Soto, D., Velez-Haro, J.M., León-Ramírez, C.G. et al. The cereal phytopathogen Sporisorium reilianum is able to infect the non-natural host Arabidopsis thaliana. Eur J Plant Pathol 153, 417–427 (2019). https://doi.org/10.1007/s10658-018-1567-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-018-1567-8