Abstract
Ascochyta blight is a devastating disease of chickpea. Breeders have been trying to introduce resistance from wild Cicer into cultivated chickpea, however, the effort is hampered by the frequent genetic drag of undesirable traits. Therefore, this study was aimed to identify potential markers linked to plant growth habit, ascochyta blight resistance and days to flowering for marker-assisted breeding. An interspecific F2 population between chickpea and C. reticulatum was constructed to develop a genetic linkage map. F2 plants were cloned through stem cuttings for replicated assessment of ascochyta blight resistance. A closely linked marker (TA34) on linkage group (LG) 3 was identified for plant growth habit explaining 95.2% of the variation. Three quantitative trait loci (QTLs) explaining approximately 49% of the phenotypic variation were found for ascochyta blight resistance on LG 3 and LG 4. Flowering time was controlled by two QTLs on LG3 explaining 90.2% of the variation. Ascochyta blight resistance was negatively correlated with flowering time (r = −0.22, P < 0.001) but not correlated with plant growth habit.
Similar content being viewed by others
Introduction
Chickpea, Cicer arietinum L., is the third most important cultivated grain legume in the world after dry bean (Phaseolus vulgaris L.) and field pea (Pisum sativum L.) (FAO 2006). Chickpea grain yield can fluctuate considerably due to a combination of biotic and abiotic stresses resulting in frequent crop failures in many regions (Knights and Siddique 2002). Since genetic diversity among chickpea germplasm is limited, chickpea breeders are prompted to search for new sources of resistance to biotic stresses in wild relatives (Abbo et al. 2003).
Ascochyta blight, caused by Ascochyta rabiei (Pass.) Labr., is a serious fungal disease of chickpea attacking all aerial parts of the plant at any growth stage, causing complete crop failure in severely affected fields (Nene 1984). Polygenic inheritance of ascochyta blight resistance has been reported in chickpea (Collard et al. 2003; Danehloueipour et al. 2007; Flandez-Galves et al. 2003; Pande et al. 2005; Santra et al. 2000, Tar’an et al. 2007). Several studies indicated that linkage group 4 has a significant effect on the reaction of chickpea to ascochyta blight whereas other genomic regions contribute minor effects (Cho et al. 2004; Iruela et al. 2006; Santra et al. 2000; Tekeoglu et al. 2002; Udupa and Baum 2003).
The number of days to flowering is an important trait for crop adaptation and productivity, especially under dryland farming systems that experience terminal drought conditions. Therefore, the ability to manipulate flowering time is an essential component of chickpea improvement (Kumar and Abbo 2001). There are limited reports on quantitative trait locus (QTL) mapping of days to flowering and plant growth habit in chickpea. The association of days to flowering and ascochyta blight resistance has been investigated in intraspecific recombinant inbred lines (RILs) (Lichtenzveig et al. 2006) and its early generations (Lichtenzveig et al. 2002). However the relationship between ascochyta blight resistance and plant growth habit is yet to be investigated in chickpea using molecular genetic tools.
Chickpea growth habit can be erect to semi-erect (cultivated type) or semi-spreading to prostrate (wildtype) based on the angle of the branches from the vertical axis, (Kumar et al. 2003). Chickpea breeders are always aiming at erect to semi-erect types for their new cultivars but they suffer from the genetic drag of undesirable traits such as spreading or prostrate growth habit and late flowering in exploiting wild Cicer species in their breeding programs; therefore, knowledge of inheritance and linkage location of these two domestication traits, and their relationships with ascochyta blight resistance, would be very useful for chickpea improvement.
Chickpea accession ICC 3996 has previously been identified as a source of resistance to ascochyta blight disease within cultivated desi chickpea germplasm (Chen et al. 2004; Collard et al. 2001; Coram and Pang 2005). Cicer reticulatum accession ILWC 184 was selected as a contrasting parent due to it being highly susceptible to ascochyta blight disease (Danehloueipour et al. 2007). These two accessions were used as parents in a QTL mapping analysis to investigate the genetic control of ascochyta blight resistance, days to flowering and plant growth habit in these parental genomes. Due to the low marker polymorphism that exists within C. arietinum, a linkage map for Cicer was generated using an interspecific cross between C. arietinum and its wild relative C. reticulatum.
Materials and methods
Plant material and mapping population
An interspecific cross was made between chickpea accession ICC 3996 (resistant to ascochyta blight, early flowering and semi-erect growth habit) and C. reticulatum accession ILWC 184 (susceptible to ascochyta blight, late flowering and prostrate growth habit). In order to obtain large numbers of F2 seeds, stem cuttings were taken from a single F1 plant following the method described by Danehloueipour et al. (2006); 390 seeds were obtained by self-pollination of eight F1 clones derived from a single mother F1 plant. Three hundred and six F2 seeds were sown in 25 cm pots with sterile potting mix in a glasshouse facility with natural daylight (12 h ± 0.5) and a temperature of 20 ± 2°C/16 ± 2°C (day/night) at The University of Western Australia (Latitude: 31°58′ S, Longitude: 115°49′ E).
The above stem cutting method was used to propagate four plants from each F2 and parental genotypes for replicated disease evaluation under glasshouse conditions.
Inoculum preparation
Ascochyta rabiei was isolated from infected chickpea stems collected from Medina field station, Western Australia in 2005. At present, according to Chongo et al. (2004), it appears that only pathotype II exists in Australia. To isolate the fungus from infected tissues, the method described by Rhaiem et al. (2007) was used. Single conidia isolate (ArIIN5-1) was cultured on V8 juice agar medium (200 ml V8 juice, 3 g CaCO3, 20 g/l Difco agar) for two weeks. Conidia were collected by flooding cultures with sterile distilled water and gently rubbing with a sterile glass rod. The concentrations of conidia were determined with a haemocytometer and adjusted to 5 × 105 pycnidiospores ml−1.
Phenotypic assessment of ascochyta blight resistance, days to flowering and plant growth habit
One-month-old clonal plants from stem cuttings were used for disease assessment. Plants were inoculated in an enclosed plastic container with the dimension of 35 × 210 × 35 cm inside a controlled environment room (15/18°C night/day, 14 h photoperiod and 75% relative humidity). Parental and F2 plants (four clonal replicates from each genotype) were inoculated with Ascochyta rabiei suspension derived from a single spore culture with a concentration of 5 × 105 pycnidiospores ml−1 to run-off. Chickpea cultivar Lasseter (susceptible to ascochyta blight) was used as a control to estimate the uniformity of disease severity. Plants were covered in the container for 48 h for the establishment of infection before replacement in the environmentally controlled room for disease development. A completely randomised block design was used.
Inoculated plants were assessed for ascochyta blight disease two weeks after inoculation using a 1–9 scale (Chen et al. 2004; Reddy and Singh 1984) as follows: (1) healthy plant, no disease; (2) lesions present, but small and inconspicuous; (3) lesions easily seen, but plant is mostly green; (4) severe lesions clearly visible; (5) lesions girdle stems, most leaves show lesions; (6) plant collapsing, tips die back; (7) plant dying, but at least three green leaves present; (8) nearly dead plant (virtually no green leaves) but still with a green stem; and (9) dead plant (almost no green parts visible).
All 306 original F2 plants and 8 plants from parental lines were measured for days to flowering (days to first flower from sowing) and plant growth habit using two categories, semi-erect (2) and prostrate (5) based on IBPGR et al. (1993).
DNA extraction and microsatellite-marker analysis
Young leaves were collected from two-month old parental, F1 and F2 plants. DNA extraction was conducted using Nucleon Phytopure Plant DNA Extraction Kit (Amersham Pharmacia Biotech) with 0.2 mg of fresh leaf material. Microsatellite primers developed by Winter et al. (1999), Huettel et al. (1999) and Lichtenzveig et al. (2005) were used to genetically characterise the F2 population.
Polymerase chain reaction (PCR) was performed in 25 μl volumes using a thermal cycler (Eppendorf). The reaction mixtures contained 50 ng of genomic DNA, 0.4 μM of each primer, 1.5 mM MgCl2, 200 μM of each dNTP, 1× PCR buffer, and 1 unit of Taq DNA polymerase (Promega). The PCR thermal cycling was performed as described by Winter et al. (1999). PCR products (5 μl) were electrophoresed either in 2% agarose in 1× TBE buffer or 8% polyacrylamide gels and, in both cases, stained with ethidium bromide and visualised by UV transillumination.
Polymorphic microsatellite markers were identified by screening the two parental lines. Polymorphic markers were then used to genotype 93 randomly selected F2 individuals along with parental and F1 control samples.
Codominant marker loci were named according to the previously published marker names. Dominant markers that amplified in the maternal parent but not in the paternal parent were suffixed by ‘NP’ (null paternal) and the paternal dominant markers were suffixed by ‘NM’ (null maternal). Markers that detected an additional locus were distinguished from each other using the molecular weight of the chickpea parent allele in parentheses at the end of the locus name.
Linkage analysis and QTL detection
Segregation of microsatellite markers in the F2 population was statistically analysed for goodness of fit to the expected ratios of 1:2:1 and 3:1 by using the χ2 test. Genetic linkage mapping was conducted with the aid of MapManager QTX version 0.30 (Manly et al. 2001) using a minimum threshold LOD (logarithm of odds) score of 3 and a maximum recombination of 25%. The Kosambi mapping function was used to convert the recombination frequencies into genetic distances in centiMorgans (cM).
QTL mapping of ascochyta blight resistance, days to flowering and plant growth habit was carried out using MultiQTL software, version 2.5 (http://www.multiqtl.com/), using general interval mapping and marker restoration options. Three hypotheses were tested for QTL detection including: H0, the QTL has no effect on a trait; H1, a single QTL on a chromosome has an effect on a trait; and H2, two linked QTLs on a chromosome have an effect on a trait. By running 3000 permutation tests, two sub-models for H1 (equal or unequal variance), and four sub-models for H2 (equal or unequal variance, with or without epistasis) were tested. The model with highest LOD score was selected for QTL detection where the models were significantly different (P < 0.05). Whenever there was no significant difference between models, the simplest model with equal variance and no epistasis (in the case of two linked QTL) were selected. To estimate the parameters and their standard deviations, 5000 bootstrap repeats were run for the selected model.
Results
Phenotypic assessment of the F2 population
Reaction to Ascochyta rabiei
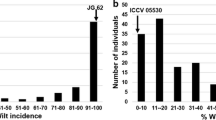
The difference among F2 plants in response to ascochyta blight disease was highly significant (P < 0.001). Based on ANOVA, 87.2% of the total variation in the population was due to genetic variation (between F2 clones) and 12.8% was due to environmental variation (within F2 clones). The resistant parent (ICC 3996) scored 3.3 (SE = 0.2) and the susceptible parent (ILWC 184) scored 6.7 (SE = 0.2). The segregation pattern of ascochyta blight reaction for the mapping population was continuous (Fig. 1).
Phenotypic distribution of 306 interspecific F2 hybrids derived from a cross between C. arietinum accession ICC 3996 and C. reticulatum accession ILWC 184. (a) response of F2 clones to Ascochyta rabiei in controlled environment conditions using a 1–9 scale, data are the mean of eight clones per F2 genotype; (b) days to flowering in the F2 population; (c) plant growth habit in the F2 population
Days to flowering
The segregation for days to flowering was continuous in the F2 population (Fig. 1). Chickpea accession ICC 3996, C. reticulatum accession ILWC 184 and their F1 hybrids flowered at 40.5 days (SE = 1.9), 74.3 days (SE = 5.1), and 52.0 days (SE = 1.7) after sowing, respectively.
Plant growth habit
The F2 population was divided into two categories for plant growth habit: 227 plants with prostrate and 79 plants with non-prostrate growth habit (Fig. 1). The parents ICC 3996 and ILWC 184 had semi-erect and prostrate growth habits, respectively. Interspecific F1 hybrids had a prostrate growth habit. Prostrate growth habit was dominant over semi-erect. Phenotypic ratio of 3 prostrate: 1 semi-erect was the best fitted model for the F2 population (χ2 = 0.108, P < 0.001).
Correlations of traits
There was a significant negative correlation between ascochyta blight resistance and days to flowering (r = −0.22, P < 0.001, n = 306). Correlation between days to flowering and plant growth habit (prostrate = 5) was highly significant (r = 0.36, P < 0.001, n = 306). There was no significant correlation between ascochyta blight resistance and plant growth habit.
General features of the genetic linkage map
The linkage map comprised 144 microsatellite markers (Fig. 2). The map had eight linkage groups covering 468.0 cM of the chickpea genome and the average spacing between microsatellite markers was 5.1 cM. Three markers remained unlinked, and markers GA26 and H1I16 were closely linked with each other but did not join other linkage groups.
Linkage map of Cicer based on the interspecific F2 population, derived from a cross between C. arietinum accession ICC 3996 and C. reticulatum accession ILWC 184, using microsatellite markers. The locations of QTLs for ascochyta blight resistance (filled square), days to flowering ( ) and a marker for plant growth habit (Prostrate) are shown on the map
) and a marker for plant growth habit (Prostrate) are shown on the map
The observed and expected marker allele segregation ratios were compared using Chi-square tests (P < 0.05). A total of 28 of the 147 markers (19%) had segregation ratios that significantly deviated from the expected 1:2:1 ratio for co-dominant markers and the 3:1 ratio for dominant markers in the F2 population. Of the 28 distorted markers, 21 were distorted towards the C. reticulatum parent (on LG3 and LG6) and seven were distorted towards heterozygotes (on LG4). There were no distorted markers towards the cultivated species parent.
Mapping traits
Plant growth habit
The plant growth habit locus, prostrate, mapped to LG3 between STMS markers TA34 (5.3 cM) and TA142 (9.2 cM). The inclusion of the prostrate locus did not result in additional double crossovers, indicating that the allocation of plants to erect or prostrate categories was accurate.
Ascochyta blight resistance
Interval mapping conducted with MultiQTL software detected three major regions significantly associated with ascochyta blight resistance on LG3 and LG4. A single QTL was detected on LG3 (LOD = 3.56, P = 0.029) explaining 21.1% of the phenotypic variation in the population (Table 1). The two-QTL model for the control of response to Ascochyta rabiei on LG4 was supported (LOD = 19.7, P < 0.001). The most appropriate model for ascochyta blight resistance on LG4 was two linked QTLs in intervals 1 and 7 (QTL 4[1] and QTL 4[7], respectively) with unequal variance and epistatic effect. These two QTLs explained 26% of the total phenotypic variation for ascochyta blight resistance (Table 2). QTL 4[1] had a major effect on disease resistance with flanking markers STMS11 (3.6 cM) and TAA170 (4.5 cM), while QTL 4[7] had a minor effect on disease resistance flanked by markers H3D09 (1.4 cM) and H1A12 (0.2 cM).
Dominance played an important role in the QTL located on LG3 which was associated with ascochyta blight resistance. Both dominant and additive effects were important in controlling the response to ascochyta blight disease in QTLs located on LG4 (Table 1).
Days to flowering
In the case of days to flowering, two QTLs were detected on LG3 (P < 0.001) with unequal variances and epistatic effect between the two QTLs. The two linked QTL model was significantly different from the single QTL model (P < 0.001). Two major QTLs on LG3, QTL 3[10] and QTL 3[13], explained 90.2% of the phenotypic variation flanked by markers TAA142 (3.2 cM) and TA64 (6.9 cM) in interval 10 and markers TS29 (7.7 cM) and TA76 (10.2 cM) in interval 13, respectively. Both additive and dominant effects were important in the control of days to flowering.
Discussion
In this study we mapped a major gene for growth habit and identified QTLs for ascochyta blight resistance and flowering time. This was the first mapping analysis for plant growth habit in chickpea, which we found to be under major gene control. Environmental variability was reduced by using clonally replicated material in controlled environmental conditions, and permitted the identification of three QTL for ascochyta blight resistance explaining approximately half of the phenotypic variation. We also found evidence that flowering time is controlled by two major genes located on the same linkage group. There was a weak negative correlation between ascochyta blight resistance and days to flowering. Early flowering was positively correlated with erectness. No correlation was found between ascochyta blight resistance and plant growth habit.
Prostrate—a major gene for plant growth habit
It was confirmed in this study that plant growth habit was under control of a single gene. Prostrate growth habit was dominant over semi-erect growth habit and this result was supported by Singh and Shyam (1959). This single gene has been mapped to a region of LG 3 flanked by STMS markers TA34 (5.3 cM) and TA142 (9.2 cM). It is the first time the map location of a major gene for growth habit has been reported for chickpea; we named the locus, Prostrate. We estimate that the accuracy of these flanking markers to predict erect/prostrate alleles in a backcross context to be at least 97.9%. This figure is based on the frequency of undetected double crossovers between markers TA34 and TA142 being the square of the recombination frequency (rf) between those markers (rf = (0.053 + 0.092)2 = 0.021). This is a conservative estimate because a proportion of double crossovers would still give a correct result if both crossovers occurred on the same side of the prostrate locus. Therefore, these markers should be useful for marker-assisted selection of the recessive erect phenotype in crosses between wild and domesticated chickpea species.
There is little previously published information available regarding the genetic basis of plant growth habit in chickpea. Using isozyme and morphological studies, Kazan et al. (1993) found a correlation between the isozyme locus Pgd-c and the locus controlling plant growth habit (Hg), which equated to an approximate genetic interval of 18 cM. The locus Pgd-c should relate to PGD6 (on LG3) in the linkage map of Winter et al. (2000) since both refer to 6-phosphogluconate dehydrogenase. Therefore, it is possible that the Prostrate locus reported here is the same as that reported by Kazan et al. (1993).
Multiple QTLs controlling ascochyta blight resistance
Polygenic inheritance for ascochyta blight resistance was found in this study. Several groups have reported QTLs for resistance to Ascochyta rabiei in chickpea, with differing numbers and strengths of QTLs, presumably due to differences in the crossing parents used, but also possibly due to differences in disease screening methods (Collard et al. 2003; Flandez-Galves et al. 2003; Santra et al. 2000, Tar’an et al. 2007).
Three QTLs for resistance to ascochyta blight explained 48.8% of the phenotypic variation. Since genetic variation explained 87.2% of the existing variation in the F2 clones, it suggests that several minor genes were also present in the population, not easily detectable with QTL mapping. The phenotypic variation explained in this study under controlled environment conditions was comparable with other studies in field conditions (Lichtenzveig et al. 2006; Tekeoglu et al. 2000) and higher than that in another study under controlled environment conditions (Flandez-Galves et al. 2003).
Our results indicate that LG4 has a significant effect on ascochyta blight resistance with two linked QTLs with peaks 12.9 cM apart, and with the domesticated parent contributing both resistance alleles. QTLs for ascochyta blight resistance on LG4 (using the standard chickpea linkage group nomenclature of Winter et al. (2000) have been reported by several researchers in both intraspecific and interspecific populations (summarised in Table 3). Lichtenzveig et al. (2006) also identified two QTLs on LG4, QTL 4.1 and QTL 4.2, with significant effects on resistance to ascochyta blight, with major (14.4%) and minor (3.8%) effects, respectively. Collard et al. (2003) reported two QTLs associated with seedling resistance to the disease, both located within LG4 about 8 cM apart. Recently, Iruela et al. (2006) identified two QTL (QTL ar1 on LG4a and QTL ar2 on LG4b) associated with resistance to ascochyta blight. Other studies (Flandez-Galves et al. 2003; Tar’an et al. 2007; Tekeoglu et al. 2002; Udupa and Baum 2003) have identified a single QTL on LG4, but the methodology of QTL analysis may not have permitted detection of a second, closely linked locus. In the current study, QTL 4[1] and QTL 4[7] had epistatic interactions and explained the largest proportion (26%) of the phenotypic variance in our study. QTL 4[1] and QTL 4[7] have a common marker with QTLs reported by Rakshit et al. (2003) (marker STMS11) and Lichtenzveig et al. (2006) (marker H1A12), respectively. Therefore, they may refer to the same QTLs. An epistatic interaction was reported earlier for the QTLs located on LG4 by Lichtenzveig et al. (2006) which is in agreement with our results. Although the QTLs in this study were found in proximity with the above mentioned QTLs in other reports, resistance was inherited differently in this population since the current resistance was recessively inherited in contrast with previous reports which showed that the resistance was dominant. This suggests that different alleles of the genes may exist in chickpea germplasm which should be considered in plant breeding programmes in order to pyramiding the resistance genes.
Another QTL identified in the current study, QTL 3[9] flanked by markers TA34 and TA142, appears to be adjacent to the QTL on LG3 found by Tar’an et al. (2007) and Kottapalli et al. (2009). Previous reports detected QTLs on LG2 (Cho et al. 2004; Udupa and Baum 2003), LG6 (Tar’an et al. 2007) and LG8 (Lichtenzveig et al. 2006) in other mapping populations but none of these were detected in this study.
The two QTLs controlling days to flowering
The inheritance of days to flowering in chickpea was controlled by two QTLs with epistatic interaction. Oligogenic inheritance of flowering time has been reported by Gumberm and Sarvjeet (1996) and Anbessa et al. (2006) who suggested that two genes controlled time to flowering. Later, Kumar and van Rheenen (2000) suggested the presence of a major gene (Efl-1/efl-1) and minor polygenes for this trait. However, Or et al. (1999) reported a single recessive major gene for time to flowering. Again these differences between reports are probably due to differences in the parents used, but environmental influences such as day length and temperature may also have differed between studies.
This study identified a novel pair of linked QTLs for days to flowering on LG3. QTL 3[10] and QTL 3[13]—flanked by markers TAA142 and TA64 in interval 10 and markers TS29 and TA76 in interval 13 of LG3, respectively—were detected with a high LOD score of 32.4 and together explained 90.2% of the phenotypic variation. Finding two linked QTLs for days to flowering was consistent with observed F2 phenotypic ratio which suggested the presence of linked duplicate dominant epistatic loci controlling the flowering time (Aryamanesh 2007). Cho et al. (2002) identified a single QTL for days to flowering on LG3 with LOD score of 3.03 flanked by markers TS57 and TA127. Although both QTLs in our study were found on LG3, their location on the linkage group was different from Cho et al. (2002) suggesting the presence of different genes for the control of flowering time. On the other hand, Lichtenzveig et al. (2006) reported two QTLs on LG2 and LG8 for days to flowering which were not detected in our population. More recently, Cobos et al. (2007) identified a QTL for days to flowering on LG4 explaining only 20% of phenotypic variation. The explained phenotypic variation in our study for days to flowering (90.2%) was much higher than in previous studies. Since phenotypic assessment for days to flowering was conducted in a controlled environment, QTLs need to be confirmed under field conditions so that they can be used more confidently in breeding programs.
Distortion towards wild parent
Deviation from the expected Mendelian ratio was observed in 19% of markers in the segregating population used in this study. Most (75%) of the distorted markers were skewed in favour of the wild parent. The rest of the distorted markers (25%) had excessive numbers of heterozygous individuals. The markers showing skewed segregation ratios were mainly located on LG3 and LG4, the linkage groups where all the QTLs identified in this study were located. Allele segregation distortion towards the wild parent have been reported in other interspecific Cicer mapping populations (Collard et al. 2003; Flandez-Galves et al. 2003; Tekeoglu et al. 2002) and may be a general feature of interspecific Cicer crosses. This skewing towards late-flowering may explain why there were no F2 individuals that flowered earlier than the chickpea parent ICC 3996, while there were many F2 individuals that flowered later than the wild parent ILWC 184.
Correlations
There was a significant negative correlation between ascochyta blight resistance and days to flowering (r = −0.22, P < 0.001). However, the efficiency was very low (R 2 = 0.048) suggesting that selection for ascochyta blight resistance based on flowering time could not result in a significant improvement of population in the breeding programmes. Similar results were obtained by Lichtenzveig et al. (2006) and Lichtenzveig et al. (2002) where they reported a significant negative correlation between these traits in the F3:4 generation of a segregating population derived from an intraspecific cross. Since most breeders are interested in early flowering chickpeas, some resistance may be lost during the selection. It was interesting to observe that no F2 individual in the population was found to be more resistant to ascochyta blight and earlier flowering than the resistant parent (ICC 3996). In contrast, most of the F2 individuals were relatively late flowering and susceptible to ascochyta blight in the range of the susceptible parent (ILWC 184).
Days to flowering was positively correlated with plant growth habit (r = 0.36, P < 0.001) in our population. Interestingly, the loci controlling days to flowering and plant growth habit were located on the same linkage group at 11.2 cM apart. No other reports of such a correlation have been reported in chickpea; however, Shah et al. (1990) reported a positive correlation between erect growth habit and early flowering in Lolium perenne.
There was no correlation between ascochyta blight resistance and plant growth habit despite co-location on the same linkage group. There are no published reports on the investigation of links between ascochyta blight resistance and plant growth habit in chickpea and wild Cicer species so far. Plant architecture can influence the microclimate within a crop (Hedley and Ambrose 1981). For instance, crop canopy affected ascochyta blight disease severity in peas; the denser the canopy, the more favorable for the disease (Porta-Puglia et al. 2000).
Clones showed the same response to ascochyta blight disease as the seed-grown chickpea plants. In this study, there was a high correlation for ascochyta blight resistance response between clonal plants derived from stem cuttings and the original plants (r = 0.98, P < 0.001, n = 8). Tar’an et al. (2007) used stem cuttings for their disease assessment but did not mention any correlation between the response of stem cuttings and original plants. Instead, they used the F3 to confirm disease measurements. Clonal plants alone were successfully used for QTL mapping of ascochyta blight resistance in this study. This experiment proved that clonal plants derived from stem cuttings can be used to phenotype plant response to ascochyta blight resistance in the F2 where several clones were tested instead of a single original plant. This technique enabled us to replicate screening without the destruction and loss of the original plants.
In conclusion, markers linked to the growth habit locus, Prostrate, and the QTLs for ascochyta blight resistance and days to flowering could be used to assist in the introgression of new allelic diversity from wild Cicer germplasm into chickpea by enabling marker-assisted retention of key characteristics in chickpea.
References
Abbo S, Berger J, Turner NC (2003) Evolution of cultivated chickpea: four bottlenecks limit diversity and constrain adaptation. Funct Plant Biol 30:1081–1087
Anbessa Y, Warkentin T, Vandenberg A, Ball R (2006) Inheritance of time to flowering in chickpea in a short-season temperate environment. J Hered 97:55–61
Aryamanesh N (2007) Chickpea improvement through genetic analysis and quantitative trait locus (QTL) mapping of ascochyta blight resistance using wild Cicer species. The University of Western Australia, Perth, WA
Chen W, Coyne CJ, Peever TL, Muehlbauer FJ (2004) Characterization of chickpea differentials for pathogenicity assay of ascochyta blight and identification of chickpea accessions resistant to Didymella rabiei. Plant Pathol 53:759–769
Cho S, Kumar J, Shultz JL, Anupama K, Tefera F, Muehlbauer FJ (2002) Mapping genes for double podding and other morphological traits in chickpea. Euphytica 128:285–292
Cho S, Chen W, Muehlbauer FJ (2004) Pathotype-specific genetic factors in chickpea (Cicer arietinum L.) for quantitative resistance to ascochyta blight. Theor Appl Genet 109:733–739
Chongo G, Gossen BD, Buchwaldt L, Adhikari T, Rimmer SR (2004) Genetic diversity of Ascochyta rabiei in Canada. Plant Dis 88:4–10
Cobos MJ, Rubio J, Fernández-Romero MD, Garza R, Moreno MT, Millán T, Gil J (2007) Genetic analysis of seed size, yield and days to flowering in a chickpea recombinant inbred line population derived from a Kabuli x Desi cross. Ann Appl Biol 151:33–42
Collard B, Ades PK, Pang ECK, Brouwer JB, Taylor PWJ (2001) Prospecting for sources of resistance to ascochyta blight in wild Cicer species. Australas Plant Path 30:271–276
Collard BCY, Pang ECK, Ades PK, Taylor PWJ (2003) Preliminary investigation of QTLs associated with seedling resistance to ascochyta blight from Cicer echinospermum, a wild relative of chickpea. Theor Appl Genet 107:719–729
Coram TE, Pang ECK (2005) Isolation and analysis of candidate ascochyta blight defence genes in chickpea. Part II. Microarray expression analysis of putative defence-related ESTs. Physiol Mol Plant Pathol 66:201–210
Danehloueipour N, Yan G, Clarke HJ, Siddique KHM (2006) Successful stem cutting propagation of chickpea, its wild relatives and their interspecific hybrids. Aust J Exp Agric 46:1349–1354
Danehloueipour N, Yan G, Clarke HJ, Siddique KHM (2007) Diallel analyses reveal the genetic control of resistance to ascochyta blight in diverse chickpea and wild Cicer species. Euphytica 154:195–205
FAO (2006) FAOSTAT DATABASE. http://faostat.fao.org/faostat/, FAO, Rome
Flandez-Galves H, Ford R, Pang ECK, Taylor PWJ (2003) An interspecific linkage map of the chickpea (Cicer arietinum L.) genome based on sequence tagged microsatellite site and resistance gene analog markers. Theor Appl Genet 106:1447–1456
Gumberm RK, Sarvjeet S (1996) Genetics of flowering time in chickpea: a preliminary report. Crop Improv 23:295–296
Hedley CL, Ambrose MJ (1981) Designing ‘leafless’ plants for improving yields of the dried pea crop. Adv Agron 34:225–277
Huettel B, Winter P, Weising K, Choumane W, Weigand F, Kahl G (1999) Sequence-tagged microsatellite site markers for chickpea (Cicer arietinum L.). Genome 42:210–217
IBPGR, ICRISAT, ICARDA (1993) Descriptors for chickpea (Cicer arietinum L.). International Board for Plant Genetic Resources, Rome, Italy; International Crops Research Institute for the Semi-Arid Tropics, Patancheru, India; International Center for Agricultural Research in the Dry Areas, Aleppo, Syria
Iruela M, Rubio J, Barro F, Cubero JI, Millan T, Gil J (2006) Detection of two QTL for resistance to ascochyta blight in an intraspecific cross of chickpea (Cicer arietinum L.): development of SCAR markers associated to resistance. Theor Appl Genet 112:278–287
Kazan K, Muehlbauer FJ, Weeden NF, Ladizinsky G (1993) Inheritance and linkage relationships of morphological and isozyme loci in chickpea (Cicer arietinum L.). Theor Appl Genet 86:417–426
Knights EJ, Siddique KHM (2002) Chickpea status and production constraints in Australia. In: Abu Bakr M, Siddique KHM, Johansen C (eds) Integrated management of Botrytis Grey mould of chickpea in Bangladesh and Australia. BARI, Gazipur, Bangladesh and Crawley, Western Australia, pp 33–41
Kottapalli P, Gaur PM, Katiyar SK, Crouch JH, Buhariwalla HK, Pande S, Gali KK (2009) Mapping and validation of QTLs for resistance to an Indian isolate of Ascochyta blight pathogen in chickpea. Euphytica 165:79–88
Kumar J, Abbo S (2001) Genetics of flowering time in chickpea and its bearing on productivity in semiarid environments. Adv Agron 72:107–138
Kumar J, van Rheenen HA (2000) A major gene for time of flowering in chickpea. J Hered 91:67–68
Kumar S, Rakshit S, Gupta S (2003) Genetics and cytogenetics of chickpea. In: Ali M, Kumar S, Singh NB (eds) Chickpea research in India. Indian institute of pulse research, Kanpur, pp 31–67
Lichtenzveig J, Shtienberg D, Zhang HB, Bonfil DJ, Abbo S (2002) Biometric analyses of the inheritance of resistance to Didymella rabiei in chickpea. Phytopath 92:417–423
Lichtenzveig J, Scheuring C, Dodge J, Abbo S, Zhang HB (2005) Construction of BAC and BIBAC libraries and their applications for generation of SSR markers for genome analysis of chickpea, Cicer arietinum L. Theor Appl Genet 110:492–510
Lichtenzveig J, Bonfil DJ, Zhang HB, Shtienberg D, Abbo S (2006) Mapping quantitative trait loci in chickpea associated with time to flowering and resistance to Didymella rabiei the causal agent of Ascochyta blight. Theor Appl Genet 113:1357–1369
Manly KF, Cudmore RH, Meer JM (2001) Map Manager QTX, cross-platform software for genetic mapping. Mamm Genome 12:930–932
Millan T, Rubio J, Iruela M, Daly K, Cubero JI, Gil J (2003) Markers associated with ascochyta blight resistance in chickpea and their potential in marker-assisted selection. Field Crops Res 84:373–384
Millan T, Clarke HJ, Siddique KHM, Buhariwalla HK, Gaur PM, Kumar J, Gil J, Kahl G, Winter P (2006) Chickpea molecular breeding: new tools and concepts. Euphytica 147:81–103
Nene YL (1984) A review of ascochyta blight of chickpea (Cicer arietinum L.). In: Saxena MC, Singh KB (eds) Ascochyta blight and winter sowing of chickpea. Martinus, Nijhoff, The Hague, Netherlands, pp 17–34
Or E, Hovov R, Abbo S (1999) A major gene for flowering time in chickpea. Crop Sci 39:315–322
Pande S, Siddique KHM, Kishore GK, Bayaa B, Gaur PM, Gowda CLL, Bertag TW, Crouch JH (2005) Ascochyta blight of chickpea (Cicer arietinum L.): a review of biology pathogenicity, and disease management. Aust J Agric Res 56:317–332
Porta-Puglia A, Bretag TW, Brouwer JB, Haware MP, Khalil SA (2000) Direct and indirect influences of morphological variations on diseases, yield and quality. In: Knight R (ed) Linking research and marketing opportunities for pulses in the 21st century. Kluwer Academic Publishers, Dordrecht, Netherlands, pp 199–220
Rakshit S, Winter P, Tekeoglu M, Munoz JJ, Pfaff T, Benko-Iseppon AM (2003) DAF marker tightly linked to a major locus for ascochyta blight resistance in chickpea (Cicer arietinum L.). Euphytica 132:23–30
Reddy MV, Singh KB (1984) Evaluation of a world collection of chickpea germplasm accessions for resistance to ascochyta blight. Plant Dis 68:900–901
Rhaiem A, Cherif M, Dyer PS, Peever TL (2007) Distribution of mating types and genetic diversity of Ascochyta rabiei populations in Tunisia revealed by mating-type-specific PCR and random amplified polymorphic DNA markers. J Phytopathol 155:596–605
Santra DK, Tekeoglu M, Ratnaparkhe M, Kaiser WJ, Muehlbauer FJ (2000) Identification and mapping of QTLs conferring resistance to ascochyta blight in chickpea. Crop Sci 40:1606–1612
Shah SG, Pearson CJ, Read JW (1990) Variability in habit, flowering and seed production within the Kangaroo Valley cultivar of Lolium perenne when grown in a range of environments. Aust J Agric Res 41:901–909
Singh D, Shyam R (1959) Genetics of two new mutants in Cicer arietinum. Indian J Genet 19:73–82
Tekeoglu M, Santra DK, Kaiser WJ, Muehlbauer FJ (2000) Ascochyta blight resistance inheritance in three chickpea recombinant inbred line populations. Crop Sci 40:1251–1256
Tekeoglu M, Rajesh PN, Muehlbauer FJ (2002) Integration of sequence tagged microsatellite sites to the chickpea genetic map. Theor Appl Genet 105:847–854
Tar’an B, Warkentin TD, Tullu A, Vandenberg A (2007) Genetic mapping of ascochyta blight resistance in chickpea (Cicer arietinum L.) using a simple sequence repeat linkage map. Genome 50:26–34
Udupa SM, Baum M (2003) Genetic dissection of pathotype-specific resistance to ascochyta blight disease in chickpea (Cicer arietinum L.) using microsatellite markers. Theor Appl Genet 106:1196–1202
Winter P, Pfaff T, Udupa SM, Huttel B, Sharma PC, Sahi S, Arreguin-Espinoza R, Weigand F, Muehlbauer FJ, Kahl G (1999) Characterization and mapping of sequence tagged microsatellite sites in the chickpea (Cicer arietinum L.) genome. Mol Gen Genet 262:90–101
Winter P, Benko-Iseppon AM, Huttel B, Ratnaparkhe M, Tullu A, Sonnante G, Pfaff T, Tekeoglu M, Santra D, Sant VJ, Rajesh PN, Kahl G, Muehlbauer FJ (2000) A linkage map of the chichpea (Cicer arietinum L.) genome based on recombinant inbred lined from a C. arietinum × C. reticulatum cross: localization of resistance genes for Fusarium wilt races 4 and 5. Theor Appl Genet 101:1155–1163
Acknowledgements
We thank Dr Peter Winter and his group for providing sample microsatellite primers and technical advice. We are grateful to Professor Fred Muehlbauer, Dr Wallace Cowling, Professor Willie Erskine and Dr Judith Lichtenzveig for critical comments on the manuscript. We also thank Ms Leila Eshraghi for technical support and Miss Nola D’Souza and Professor Richard Oliver for providing some microsatellite primers. This project was supported by the Ministry of Science, Research and Technology of Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aryamanesh, N., Nelson, M.N., Yan, G. et al. Mapping a major gene for growth habit and QTLs for ascochyta blight resistance and flowering time in a population between chickpea and Cicer reticulatum . Euphytica 173, 307–319 (2010). https://doi.org/10.1007/s10681-009-0086-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-009-0086-2