Abstract
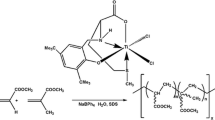
A new Titanium (IV) complex, dichlorobis(salicylato)titanium(IV) [TiL2Cl2] (1) {LH = Salicylic acid} has been synthesized and characterized by elemental analysis, 1H NMR, 13C NMR, IR and UV-visible spectroscopy. As a post-metallocene complex it’s olefin polymerization activity has been investigated at room temperature in aqueous emulsion. In presence of tetraphenylborate as cocatalyst, it has been found to exhibit moderate to high activity in the range of 103 in homopolymerization and copolymerization of styrene and methyl methacrylate to produce syndiotactic rich, ultrahigh molecular weight polymers with low molecular weight distribution. The polymer latex produced has been analyzed by DLS, HRTEM and FESEM which revealed that the latex is highly stable and contains polymer particles having size around 50 nm. The microstructures, molecular weights and thermal properties of the polymers have also been determined by 1H-NMR, 13CNMR, GPC and DSC analysis. The polymers have also been characterized by 1H-NMR, 13C-NMR, GPC and DSC analysis.













Similar content being viewed by others
References
Britovsek GJP, Gibson VC, Wass DF (1999) Angew Chem Int Ed 38:428–447
Suzuki Y, Terao H, Fujita T (2003) Bull Chem Soc Japan 76:1493–1517
Stephan DW (2005) Organometallics 24:2548–2560
Bolton PD, Mountford P (2005) Adv Synth Catal 347:355–366
Gibson VC, Redshaw C, Solan GA (2007) Chem Rev 107:1745–1776
Li T, Song W, Ai H, You Q, Zhang A, Xie G (2015) J Polym Res 22:631
Yoshida Y, Matsui S, Takagi Y et al. (2000) Chem Lett 1270–1271
Yoshida Y, Matsui S, Takagi Y, Mitani M, Nakano T, Tanaka H, Kashiwa N, Fujita T (2001) Organometallics 20:4793–4799
Yoshida Y, Saito J, Mitani M et al. (2002) Chem Commun 1298–1299
Yoshida Y, Nakano T, Tanaka H, Fujita T (2002) Isr J Chem 42:353–359
Yoshida Y, Matsui S, Fujita T (2005) J Organomet Chem 690:4382–4397
Matsugi T, Matsui S, Kojoh SI et al. (2001) Chem Lett 566–567
Matsugi T, Matsui S, Kojoh SI, Takagi Y, Inoue Y, Nakano T, Fujita T, Kashiwa N (2002) Macromolecules 35:4880–4887
Suzuki Y, Inoue Y, Tanaka H, Fujita T (2004) Macromol Rapid Commun 25:493–497
Matsui S, Tohi Y, Mitani M et al. (1999) Chem Lett 1065–1066
Matsui S, Mitani M, Saito J et al. (1999) Chem Lett 1263–1264
Matsui S, Mitani M, Saito J et al. (2000) Chem Lett 554–555
Mitani M, Saito J, Ishii S, Nakayama Y, Makio H, Matsukawa N, Matsui S, Mohri J, Furuyama R, Terao H, Bando H, Tanaka H, Fujita T (2004) Chem Rec 4:137–158
Suzuki Y, Kashiwa N, Fujita T et al. (2002) Chem Lett 358–359
Suzuki Y, Tanaka H, Oshiki T, Takai K, Fujita T (2006) Chem Asian J 1:878–887
Budagumpi S, Kimb K, Kima I (2011) Coord Chem Rev 255:2785–2809
Nakata N, Toda T, Ishii A (2011) Polym Chem 2:1597–1610
Gibson VC, Spitzmesser SK (2003) Chem Rev 103:283–315
Mecking S, Held A, Bauers FM (2002) Angew Chem Int Ed 41:544–561
Kostjuk SV, Ganachaud F (2010) Acc Chem Res 43:357–367
Ballav N, Biswas M (2006) J Polym Res 13:115–119
Maitre C, Ganachaud F, Ferreira O, Lutz JF, Paintoux Y, Hemery P (2000) Macromolecules 33:7730–7736
Limouzin C, Caviggia A, Ganachaud F, Hemery P (2003) Macromolecules 36:667–674
Rehor A, Tirelli N, Hubbell JA (2002) Macromolecules 35:8688–8693
Kobayashi M, Matsumoto Y, Uchiyama M, Ohwada T (2004) Macromolecules 37:4339–4341
Korthals B, Berkefeld A, Ahlmann M, Mecking S (2008) Macromolecules 41:8332–8338
Huber J, Mecking S (2010) Macromolecules 43:8718–8723
Claverie JP, Soula R (2003) Prog Polym Sci 28:619–662
Mecking S (2007) Colloid Polym Sci 285:605–619
Buettner KM, Valentine AM (2012) Chem Rev 112:1863–1881, and the references there in
Tinoco AD, Incarvito CD, Valentine AM (2007) J Am Chem Soc 129:3444–3454
Köpf H, Köpf-Maier P (1979) Angew Chem Int Ed 18:477–478
Toney JH, Marks TJ (1085) J Am Chem Soc 107:947–953
Berkefeld A, Mecking S (2006) Angew Chem Int Ed 45:6044–6046
Hristov IH, DeKock RL, Anderson GDW, Göttker-Schnetmann I, Mecking S (2005) Inorg Chem 44:7806–7818
De SK, Bhattacharjee M (2009) J Polym Sci Part A: Polym Chem 47:6496–6503
De SK, Bhattacharjee M (2011) J Polym Sci Part A: Polym Chem 49:3920–3927
De SK, Bhattacharjee M (2013) J Polym Sci Part A: Polym Chem 51:1540–1549
Wilkinson G, Birmingham JM (1954) J Am Chem Soc 76:4281–4284
Doyle G, Tobias RS (1967) Inorg Chem 6:1111–1115
Ishihara N, Seimiya T, Kuramoto M, Uoi M (1986) Macromolecule 19:2464–2465
Feil F, Harder S (2003) Macromolecules 36:3446–3448
Bolig AD, Chen EYX (2001) J Am Chem Soc 123:7943–7944
Hatada K (1999) J Polym Sci Part A: Polym Chem 37:245–260
Claudy P, Letoffe JM, Camberlain Y, Pascault JP (1983) Polym Bull 9:208–215
Kitayama T, Masuda E, Yamaguchi M, Nishiura T, Hatada K (1992) Polym J 24:817–827
Marshall EL, Gibson VC, Edited by Baugh LS et al. (2008) Stereoselective polymerization with single-site catalysts 593–626
Padwa AR (1989) Prog Polym Sci 14:811–833
Boffa LS, Novak BM (2000) Chem Rev 100:1479–1493
Yang XH, Liu CR, Wang C, Sun XL, Guo YH, Wang XK, Wang Z, Xie ZW, Tang Y (2009) Angew Chem Int Ed 48:8099–8102
Jensen TR, Yoon SC, Dash AK, Luo L, Marks TJ (2003) J Am Chem Soc 125:14482–14494
Faraguna F, Siuc V, Vidović E, Jukić A (2015) J Polym Res 22:245
Bajpai S, Srivastava AK (2001) J Appl Polymn Sci 80:2774–2781
Furukawa J, Tsuruta T, Inoue S, Kawasaki A, Kawabata N (1959) J Polym Sci 35:268–271
Cunningham MF, Geramita K, Ma JW et al. (2000) 41:5385–5392
Acknowledgments
We thank Prof. Manish Bhattacharjee, Department of Chemistry, IIT Kharagpur for allowing us to use GPC facility in his lab.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 3053 kb)
Rights and permissions
About this article
Cite this article
Sharma, K., Lunawat, G. & De, S.K. Environmentally benign stereoselective polymerizations of polar as well as nonpolar olefins by a new postmetallocene Ti(IV) salicylate complex at ambient temperature in aqueous emulsion. J Polym Res 23, 41 (2016). https://doi.org/10.1007/s10965-016-0924-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-016-0924-6