Abstract
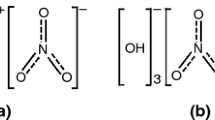
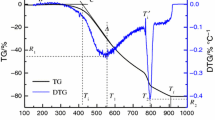
Characterized with low burning temperature and low concentration of toxic gases, the azodicarbonamide (ADC), basic cupric nitrate (BCN) and 10 % metal oxide (CuO, ZnO, MnO2, Fe2O3) gas-generating agents have been investigated. The thermal analysis results show that the thermal decomposition process of ADC/BCN/metal oxide is similar to that of ADC/BCN, and the addition of metal oxides makes the initial decomposition temperature decrease. Among the ADC/BCN/metal oxide mixtures that were examined, ADC/BCN/CuO mixture exhibits a better burning performance. The maximum burning temperature (735 °C) and combustion heat (1900.5 J g−1) have a relatively low value, and the content of CO is the lowest (8390 ppm).





Similar content being viewed by others
References
Kirchoff GF, Schneiter FE. Pelletizable, rapid and cool burning solid nitrogen gas generate. US Patent 4203787; 1980.
Munich S Z, Deisenhofen WH. Gas-generating composition. US Patent 4834817; 1989.
Catwright R V. Crash bag propellant composition and method for generating nitrogen gas. US Patent 4929290; 1990.
Ramaswamy CP, Souriraja PR. Gas generating composition for air bags. US Patent 5089069; 1992.
Ube YI, Kitakyushu KI, Yamaguchi MM. Gas generating composition for automobile air bag. US Patent 5178696; 1993.
Chen S, Cheng Y. Study on composition of sodium azide gas generan. Initiat Pyrotech. 2001;4:37–9.
Chang S, Lamm SH. Human health effects of sodium azide exposure: a literature review and analysis. Int J Toxicol. 2003;22(3):175–86.
Trout D, Esswein EJ, Brown K, Solomon G, Miller M. Exposure and health effects: an evaluation of workers at sodium azide production. Am J Ind Med. 1996;30(3):343–50.
Hongshe W. Study on nitrogen-rich based gas generating pyrotechnic compositions. Ph.D. dissertation, Beijing: Beijing Institute of Technology; 2005.
Qamirani E, Razavi HM, Wu X, Davis MJ, Kuo L, Hein TW. Sodium azide dilates coronary arterioles via activation of inward rectifier K+ channels and Na+–K+–ATPase. Am J Physiol Heart Circ Physiol. 2006;290(4):H1617–23.
Xiande Y. Productions and applications of azodicarbonamide foaming agent in China. Chem Propellants Polym Mater. 2004;1:44–48.
Oxley JC, Smith JL, Naik S, Moran J. Decompositions of urea and guanidine nitrates. J Energ Mater. 2009;27:17–39.
Takashi K, Yoshida T. The combustion characteristic of ADCA. Gunpower Soc. 1995;56(6):249.
Hara K, Yoshida T. Proceedings of the 20th international pyrotechnics seminar. Colorado Springs, USA, 9; 1994.
Hara Kazuo, Yoshida Tadao. Concept and performance of a non-azide propellant for automotive. Propellants Explos Pyrotech. 1998;23:28–33.
Jianzhou W. Gas-generating agent composition. CN Patent 200410086968.7; 2003.
Jianzhou W. Gas-generating agent composition. CN Patent 03801083.6; 2003.
Yuping Li. The study of a new gas generating composition for automobile safety airbag. Master dissertation, North University of China; 06-01, 2010.
Matsuoka Ichi, Toyama. The gas generating composition. CN Patent 95192492.3. 1995.
Li Yanchun, Cheng Yi, Hui Yun-Long, Yan Shi. The effect of ambient temperature and boron content on the burning rate of the B/Pb3O4 delay compositions. J Energ Mater. 2010;28:77–84.
Reed RA. The kinetics and mechanism of the thermal decomposition of azodicarbonamide. Br Plast. 1960;33(10):468–72.
Mei Xinliang, Cheng Yi, Li Yanchun. Thermal decomposition properties of guanidine nitrate and basic cupric nitrate. J Therm Anal Calorim. 2013;114:131–5.
Hao J, Yu J, Bao G, Che J. Pre-ignition reactions mechanism of B/BaCrO4 delay composition. Initiat Pyrotech 2006;3:27–9.
Jie Zhang, Ling Shi, JunYing Zhang. The mechanism of thermal decomposition of azodicarbonamide and the influence of zinc oxide. Natural Science. 2011;38(3):39–42.
Akiyoshi Miyako, Nakamura Hidetsugu, Hara Yasutake. The strontium complex nitrates of carbohydrazide as a non-azide gas generator for safer driving-the thermal behavior of the Sr complex with various oxidizing agents. Propellants Explos Pyrotech. 2000;25:224–9.
Acknowledgements
This work was supported by the Fundamental Research Funds for the National Natural Science Foundation of China (NSFC51202113) and Central Universities (NUST 2011 YBXM10).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, H., Xu, T., Zhu, X. et al. Design and study of ADC/BCN/metal oxide gas-generating agents. J Therm Anal Calorim 123, 75–80 (2016). https://doi.org/10.1007/s10973-015-4858-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4858-8