Abstract
Breast cancer is the most common female cancer in women, and its estrogen receptor (ER)-negative subtype (ENBC) and triple-negative subtype (TNBC) have unfavorable prognosis in comparison with ER-positive subtype. MiRNAs are small noncoding RNAs that bind to the 3′-UTR region of targeting mRNAs to regulate gene expression. Mir-519d-3p was found to be associated with breast cancer for its potential role in proliferation and metastasis. To explore its potential role and mechanism of miR-519d-3p in breast carcinogenesis, we determined whether miR-519d-3p regulates breast cancer cell proliferation and motility by performing wound-healing assays and migration–invasion assays. We found that miR-519d-3p significantly inhibits proliferation and motility of ENBC and TNBC cells. Overexpression of miR-519d-3p arrested breast cancer cells in the G0/G1 phase and reduced the expression of CDK4, 6/Cyclin D1, and CDK2/Cyclin E1. It was reported that miR-519d-3p or miR-519d-3p expression was associated with cancer metastasis and clinical staging. Since LIM domain kinase 1 (LIMK1) was highly expressed in breast cancer and a major regulator of breast cancer growth and metastasis, we further demonstrated that LIMK1 is a potential target of miR-519d-3p by dual-luciferase report assay. Mir-519d-3p decreases LIMK1 expression at mRNA and protein levels, and the protein level and phosphorylation of cofilin 1 (CFL1), one of the key downstream targets of LIMK1. Our findings suggest that miR-519d-3p regulates the LIMK1/CFL1 pathway in breast cancer and this new venue could be targeted for future breast cancer therapy.
Similar content being viewed by others
Introduction
It has been estimated that there are approximately 1.7 million new breast cancer cases diagnosed yearly in the world, with about 170,000 yearly new cases in China and over 40,000 death roll [1] and approximately 250,000 yearly new cases and more than 40,000 deaths in USA in 2017 [2]. To improve therapeutic outcomes and ultimate prognosis for breast cancer, key mechanisms that regulate breast cancer development, progression, and metastasis are urgently sought. The microRNAs (miRNAs), a class of small noncoding RNAs of about 22 nt that regulate gene expression by binding to the 3′-UTR region of mRNA to inhibit or degrade target mRNAs to impact oncogenes or tumor suppressor genes [3], are important regulators that function in a variety of biological processes, such as cancer cell proliferation, tumor development, and metastasis [4].
LIM kinase 1(LIMK1) is a serine/threonine kinase that plays a key role in actin and microtubule dynamics through phosphorylating cofilin (CFL1) which is widely thought as an intracellular actin-modulating protein [5]. It has been reported that LIMK1 is highly expressed in some cancers, including colorectal and ovarian cancers [6, 7], to promote tumor progression, and silencing of LIMK1 significantly inhibits cancer metastasis and progression. A number of studies also confirmed that LIMK1 plays a major role in breast cancer growth, metastasis, and progression [8,9,10]. Therefore, targeting LIMK1 may represent a potential therapeutic strategy for treating breast cancer.
It has also been reported that miRNAs could target LIMK1 to inhibit cancer progression in different cancers. For instance, in anaplastic thyroid cancer and cutaneous squamous cell carcinoma, miR-20a could target LIMK1 to inhibit cancer proliferation and metastasis [11, 12]; miR-138 could inhibit migration and invasion in ovarian and lung cancer by directly targeting the LIMK1 and LIMK1/CFL1 pathways [13, 14]. In breast cancer, it was reported that miR-143-3p targets LIMK1 and suppresses the progression of TNBC cells [15]. We have recently reported that miR-200b-3p/miR-429-5p could also inhibit breast cancer metastasis by targeting the LIMK1/CFL1 pathway [16].
In the present study, we aim to determine whether miR-519d-3p also targets the LIMK1 pathway to inhibit breast cancer growth and metastasis. Our study revealed that the overexpression of miR-519d-3p significantly inhibits proliferation, migration, and invasion of ENBC and TNBC cells. Meanwhile, overexpression of miR-519d-3p arrests breast cancer cells at the G0/G1 phase by decreasing the expression of CDK2/Cyclin E1, CDK4, and CDK6/Cyclin D1 complexes. Our results demonstrate that miR-519d-3p regulates the LIMK1 pathway; this new information may provide a novel avenue of therapeutic option by targeting miR-519d-3p–LIMK1–CFL1 to treat breast cancer, particularly for patients with ENBC and TNBC.
Materials and methods
Cell culture and transfection
MDA-MB-231 and HCC1937 breast cancer cell lines were purchased from the Chinese Academy of Science at Shanghai (Shanghai, China). HEK 293T human embryonic kidney cells were kindly a gift from the Department of Laboratory Medicine, Shanghai Tenth People’s Hospital. These cell lines were cultured in Dulbecco’s Modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) with 10% fetal bovine serum (FBS, Gibco, Grand Island, NY) and 1% penicillin–streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in an incubator at 37 °C in 5% CO2.
MiR-519d-3p mimics and negative-control (NC) mimics were purchased from Ibsbio (Shanghai, China). The sequence of the miR-519d-3p mimics was sense, 5′-CAAAGUGCCUCCCUUUAGAGUG-3′; antisense, 5′-CUCUAAAGGGAGGCACUUUGUU-3′. The NC mimic sequence was sense, 5′-UCACAACCUCCUAGAAAGAGUAGA-3′; antisense, 5′-UACUCUUUCUAGGAGGUUGUGAUU-3′ (NC mimics as the control). MDA-MB-231 and HCC1937 cells (2.5 × 105/well) were cultured in a 6-well plate until the cells reached 40–50% confluence. Then the miR-519d-3p mimics were transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) into cells according to the manufacturer’s instructions. The concentration of mimics used was 100 nM/L, and the ratio of mimics to Lipofectamine 2000 was 5:4 (volume).
Colony formation assay
MDA-MB-231 cells were transfected with 100 nM miR-519d-3p mimics or NC mimics for 24 h in 6-well plate. Single-cell suspensions of the transfected cells were prepared, and the cells were re-plated in a 12-well plate at a density of 600 cells/well. The culture medium was changed every 3 days. After 2 weeks, the surfaces of 12-well plate were washed twice with phosphate-buffered saline (PBS), then treated with 4% polyformaldehyde for 10 min, stained with 0.1% crystal violet for 10 min, and washed with double-distilled water three times. Photos of the culture surfaces on dried plates were taken.
3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) proliferation assay
For MTT proliferation assay, MDA-MB-231 cells (3 × 103/well) were seeded in a 96-well plate. The cell proliferation rate was determined by measuring the absorbance according to the manufacturer’s instructions (Thermo, USA) at 24, 48, and 72 h. A microplate spectrophotometer (BioTek, Winooski, VT, USA) was used to measure the absorbance OD values of each sample at 490 nm.
Cellular motility assays
After MDA-MB-231 cells were transfected with miR-519-3p or NC mimics, transwell assays were performed to evaluate cellular migration and invasion, with or without Matrigel. After treating MDA-MB-231 cells with miR-519d-3p mimics, or NC mimics, 5 × 104 cells/well were seeded in the upper chambers of a 24-well Transwell plate (Corning, NY, USA) with 200 μL of DMEM supplemented with 0.1% bovine serum albumin (BSA). The lower chambers were filled with 600 μL of DMEM with 10% FBS. The cells were cultured for 12–16 h, and then the outer surface of each insert was washed three times with PBS and fixed stained for 10 min as described in colony formation assay. Photos of the bottoms of the inserts were captured when the surface had dried.
Wound-healing assay
After MDA-MB-231 cells were transfected with miR-519-3p or NC mimics, the cells were cultured to reach 90% confluence, and then the plate containing cells was scratched with a 200-μL pipette tip. Photos of the cells in culture were taken at 0 and 24 h from the same position to assess cell motility via observing the area of blank.
Quantitative reverse transcription polymerase chain reaction
MDA-MB-231 and HCC1937 cells were treated with miR-519d-3p mimics or NC mimics in 6-well plates. According to the manufacturer’s instructions of TRIzol reagent (Invitrogen), the cells were collected 36 h later for total RNA isolation and cDNA was generated using a real-time polymerase chain reaction (PCR) kit (Takara, Shiga, Japan). Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed on a 7900HT Fast real-time PCR system (Applied Biosystems, Singapore). We followed our previously published amplification procedure: 5 min at 95 °C, followed by 40 cycles at 95 °C for 30 s and 65 °C for 45 s. Expression of mRNA was assessed by evaluating threshold cycle values. The threshold cycle values of LIMK1 were normalized to the level of GAPDH expression. The primer sequences used were as follows [15]: LIMK1-forward: 5′-CAAGGGACTGGTTATGGTGGC-3′; LIMK1-reverse: 5′-CCCCGTCACCGATAAAGGTC-3′; GAPDH-forward: 5′-CATGAGAAGTATGACAACAGCCT-3′; and GAPDH-reverse: 5′-AGTCCTTCCACGATACCAAAGT-3′. The qRT-PCR results were analyzed using the 2−ΔΔt method [17].
Western blot analysis
MDA-MB-231 and HCC1937 cells were collected 72 h after treatment with miR-519d-3p and NC mimics, and total protein samples were extracted from them using a RIPA buffer (Beyotime Biotechnology, Shanghai, China). Protein concentrations of the samples were measured with a BCA Protein Assay Kit (Beyotime Biotechnology) according to the manufacturer’s instructions. Total protein samples (60 μg) were added to each pore to perform the Western blot assay as in our previous work [15]. After three washes with phosphate-buffered saline with Tween 20, immunoreactive protein bands were detected using an Odyssey Scanning System (LI-COR Biosciences, Lincoln, NE, USA). The antibodies used were as follows: PCNA (1:2000; Cell Signaling Technology, Danvers, MA, USA), MMP2 (1:1000; Arigo Biolaboratories, Hsinchu City, Taiwan, China), MMP9 (1:1000; Arigo Biolaboratories), LIMK1 (1:500; Arigo Biolaboratories), CFL1 (1:500; Arigo Biolaboratories), phospho-CFL1 (1:500; Arigo Biolaboratories), CDK2 (1:2000; Bioworld, Shanghai, China), CDK4 (1:2000; Abcam, Cambridge, MA, USA), CDK6 (1:2000; Abcam), cyclin D1 (1:10 000; Abcam), cyclin E1 (1:2000; Abcam), β-actin (1:2000; Santa Cruz Biotechnology, CA, USA), and anti-rabbit or anti-mouse secondary antibody (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Cell cycle assay
After treating MDA-MB-231 cells with miR-519d-3p mimics or NC mimics for 36 h as previously described, the cells were collected, washed, suspended in PBS, and fixed in cold 70% ethanol overnight at 4 °C. After a 30-min digestion with RNase (0.1 g/L), 300 μL (0.05 g/L) of propidium iodide staining solution was added to each cell sample. The stained samples were incubated for 30 min at room temperature in the dark. Cell cycle progression was then analyzed using flow cytometry.
Dual-luciferase reporter assay
HEK 293T cells were used in dual-luciferase report assays. Firstly, HEK293T were seeded in 48-well plates and cultured until they reached 70% confluence. The psiCHECK-2/LIMK1 3′-untranslated region (UTR) and psiCHECK-2/LIMK1 3′-UTR mutant reporter plasmids were purchased from Ibsbio (Shanghai, China). HEK 293T cells were transiently co-transfected with 0.2 µg psiCHECK-2/LIMK1 3′-UTR wild reporter plasmids (wild type 1 and wide type 2) or psiCHECK-2/LIMK1 3′-UTR mutant reporter plasmids (mutant type 1 and mutant type 2) together with 100 nM/L miR-519d-3p mimics, or NC mimics using Lipofectamine 2000. After 72 h, cell lysates were collected to determine firefly and Renilla luciferase activity using a Dual Luciferase Assay Kit (Beyotime Biotechnology, Shanghai, China). Firefly luciferase activity values were normalized to the values for Renilla luciferase, and the results are presented as the ratio of firefly to Renilla activity values.
Statistical analysis
Data were collected from at least three separate experiments and presented as the mean ± standard (mean ± SD). One-way ANOVA test or Student’s t test was used for comparisons between groups. Differences were considered significant for p values less than 0.05. GraphPad Prism version 6.0 (GraphPad, San Diego, CA, USA) was used to perform all statistical analyses.
Results
Mir-519d-3p suppresses proliferation of ENBC and TNBC cells
We overexpressed miR-519d-3p in breast cancer cells to determine its impact on cellular growth. Overexpression of miR-519d-3p significantly inhibited the colony formation ability of MDA-MB-231 cells (Fig. 1a), in a time-dependent way (Fig. 1b). We examined the proliferation biomarker PCNA protein level in representative TNBC cell line MDA-MB-231 and ENBC cell line HCC1937. Both cells showed decreased PCNA expression after overexpression of miR-519d-3p (Fig. 1c, d). These results suggest that the overexpression of miR-519d-3p suppressed breast cancer cell proliferation.
MiR-519d-3p suppresses the proliferation of breast cancer cells. a Overexpression of miR-519d-3p suppressed colony formation of MDA-MB-231 cells; b MTT assay showed the overexpression of miR-519d-3p suppressed the proliferation of MDA-MB-231 cells in a time-dependent manner; c overexpression of miR-519d-3p decreased the protein expression of PCNA in MDA-MB-231 cells, Student’s t test, *** means p < 0.001; d overexpression of miR-519d-3p decreased protein expression of PCNA in HCC1937 cells, Student’s t test, ** means p < 0.01
Mir-519d-3p suppresses migration and invasion of breast cancer cells
Motility assays and wound-healing assays were performed to detect motility changes in TNBC cell line MDA-MB-231. Transfecting with miR-519d-3p mimics, migration of MDA-MB-231 was significantly inhibited (Fig. 2a). Meanwhile, overexpression of miR-519d-3p also inhibited the invasion ability of MDA-MB-231 cells as measured in Matrigel assays (Fig. 2b). As shown in Fig. 2c, compared with the NC control group, wound-healing assay showed that the overexpression of miR-519d-3p suppressed MDA-MB-231 cell migration. It was reported that MMP2 and MMP9 are important regulators in cancer metastasis and could serve as metastatic biomarkers [18]. We detected MMP2 and MMP9 proteins to index whether miR-519d-3p inhibits migration and invasion through decreasing these regulators. As shown in Fig. 2d, e, Western blot clearly showed that the overexpression of miR-519d-3p decreased MMP2 and MMP9 in MDA-MB-231 and HCC1937 cells. Our results demonstrate that miR-519d-3p suppresses the motility of ENBC and TNBC cells.
MiR-519d-3p suppresses migration and invasion of breast cancer cells. a Transwell assay showed the overexpression of miR-519d-3p in suppressing the migration of MDA-MB-231 cells, Student’s t test, ** means p < 0.01; b transwell assay showed that the overexpression of miR-519d-3p suppressed the invasion of MDA-MB-231 cells, Student’s t test, *** means p < 0.001; c wound-healing assay showed that the overexpression of miR-519d-3p suppressed the motility of MDA-MB-231 cells; d overexpression of miR-519d-3p decreased the protein expression of MMP2 and MMP9 in MDA-MB-231 cells, Student’s t test, *** means p < 0.001; e overexpression of miR-519d-3p decreased the protein expression of MMP2 and MMP9 in HCC1937 cells, Student’s t test, ** means p < 0.01, *** means p < 0.001
Mir-519d-3p arrests breast cancer cells into G0/G1 cell cycle
After transfecting with miR-519d-3p mimics for 36 h, MDA-MB-231 cells were collected for analyzing changes in cell cycle progress. We performed flow cytometry analysis and found that the overexpression of miR-519d-3p in MDA-MB-231 cells significantly arrested the cells into the G0/G1 phase, compared with the NC group (Fig. 3a). To further explore potential molecular mechanism, we detected several proteins that related to G0/G1 cell cycle. As shown in Fig. 3b, c, overexpression of miR-519d-3p significantly decreased the protein levels of CDK2/CCNE1, CDK4/6, and CCND1 in both MDA-MB-231 and HCC1937 cells. All these results showed that the overexpression of miR-519d-3p modulates cell cycle progression and pushes breast cancer cells into the G0/G1 phase.
MiR-519d-3p arrests breast cancer cells into the G0/G1 cell cycle phase. a Overexpression of miR-519d-3p arrested MDA-MB-231 cells into the G0/G1 cell cycle phase, Student’s t test, * means p < 0.05; b overexpression of miR-519d-3p decreased the protein expression of CDK2/CCNE1 and CDK4, 6/CCND1 compounds in MDA-MB-231 cells, Student’s t test, ** means p < 0.01, *** means p < 0.001. c Overexpression of miR-519d-3p decreased the protein expression of CDK2/CCNE1 and CDK4, 6/CCND1 compounds in HCC1937 cells, Student’s t test, * means p < 0.05, *** means p < 0.001
Mir-519d-3p targets LIMK1 and decreased LIMK1 expression in breast cancer cells
We searched databases including TargetScan, miRbase, and miRwalk and found that LIMK1 is a target gene of miR-519d-3p. These databases showed that there were two potential binding sites for the LIMK1 3′-UTR region and miR-519d-3p. To confirm whether LIMK1 was a target of miR-519d-3p and explore potential binding sites, specific plasmids containing wild-type binding sites and mutant-binding sites of the LIMK1 3′-UTR region were constructed. As shown in Fig. 4a, miR-519d-3p decreased luciferase activity in both wild-type and mutant groups in which both vectors contain binding site covering position 660–666, suggesting that this was not a binding site of LIMK1 and miR-519d-3p; Fig. 4b shows that miR-519d-3p inhibited luciferase activity in the wild-type but not that in the mutant vectors, demonstrating that miR-519d-3p could target LIMK1 in the position of 1076–1082 at the 3′-UTR region of LIMK1. We further determined whether miR-519d-3p inhibits LIMK1 expression. As shown in Fig. 5a, b, we found that the overexpression of miR-519d-3p inhibited mRNA level of LIMK1 in MDA-MB-231 and HCC1937 cells. This was further validated by Western blot where the overexpression of miR-519d-3p decreased LIMK1 protein level and expression and phosphorylation of CFL1, a direct downstream target of LIMK1, in MDA-MB-231 and HCC1937 cells (Fig. 5c, d). All these results showed that miR-519d-3p targets the LIMK1 pathway by suppressing the expression and activation of LIMK1 and CFL1.
LIMK1 is a direct target of miR-519d-3p in breast cancer. a Binding position of 660–666 is not a binding site for miR-519d-3p and LIMK1, Student’s t test, ** means p < 0.01, *** means p < 0.001; b binding position of 1076–1082 is a binding site for miR-519d-3p and LIMK1, Student’s t test, * means p < 0.05
MiR-519d-3p suppresses the expression of LIMK1 and its substrate in breast cancer cells. a Overexpression of miR-519d-3p decreased the mRNA expression of LIMK1 in MDA-MB-231 cells, Student’s t test, *** means p < 0.001; b overexpression of miR-519d-3p decreased the mRNA expression of LIMK1 in HCC1937 cells, Student’s t test, *** means p < 0.001; c overexpression of miR-519d-3p decreased the protein expression of the LIMK1 and LIMK1/CFL1 pathways in MDA-MB-231 cells, Student’s t test, ** means p < 0.01, *** means p < 0.001; d overexpression of miR-519d-3p decreased the protein expression of the LIMK1 and LIMK1/CFL1 pathways in HCC1937 cells, Student’s t test, ** means p < 0.01, *** means p < 0.001
LIMK1 is highly expressed in breast cancer
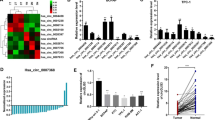
We examined the expression of LIMK1 in breast cancer through Oncomine website (https://www.oncomine.org/). The TCGA database (Fig. 6a) showed that LIMK1 was highly expressed in invasive ductal breast cancer (fold change: 2.340, p value: 1.03E−18); in particular, the expression of LIMK1 was especially higher in TNBC samples (Fig. 6b, fold change: 1.265, p value: 0.01). These results support a key role for LIMK1 mammary carcinogenesis and an aggressive phenotype.
Discussion
In our study, we found that the overexpression of miR-519d-3p significantly suppresses the proliferation of ENBC and TNBC cells; this was supported by the detection of PCNA and Ki67 expression, which are typical biomarkers of cellular proliferation [19]. Meanwhile, overexpression of miR-519d-3p significantly decreased the motility of MDA-MB-231 breast cancer cells in terms of migration and invasion. Furthermore, the expression of MMP2 and MMP9, typical markers for metastasis, was also suppressed by overexpressing miR-519d-3p. We also show that the enhanced miR-519d-3p expression arrests MDA-MB-231 cells into the G0/G1 phase by decreasing the expression of CDK2/Cyclin E1 and CDK2, CDK4/Cyclin D1 complexes. These findings demonstrate that miR-519d-3p is a potential tumor suppressor of breast cancer cells, consistent with literature that miR-519d mediates the downregulation of STAT3 and suppresses breast cancer progression [20]. Furthermore, miR-519d-3p was reported to be involved in cisplatin-associated resistance in breast cancer stem cells by downregulating the expression of MCL-1 [21].
It is documented that LIMK1 regulates the tumor cell invasion and matrix degradation in MDA-MB-231 breast cancer cells [22] and knockdown of LIMK1 with specific LIMK1 inhibitors causes significantly the inhibition of migration, invasion, and progression of breast cancer [10, 23]. It is well known that ROCK1 is a upstream target of LIMK1 and it phosphorylates and activates LIMK1, which in turn phosphorylates CFL1 to suppress actin-depolymerizing activity and regulate subsequent biological function of cells (https://www.ncbi.nlm.nih.gov/gene/6093). It was reported that plumbagin reduces A549 lung cancer cells’ invasion through inhibiting the ROCK1/LIMK1 signaling pathway to suppress osteopontin-induced lung metastasis in BalB/c mice [24]. In addition, ROCK1 could also regulate LIMK1 to regulate growth, maturation, and other actin-related functions in mast cells [25].
MiRNAs have been extensively reported to play important roles in many human diseases, including myocardial infarction and cardiovascular diseases, diabetes, obesity, and cancer [26]. For example, miR-519d was shown to specifically and dose-dependently suppress the translation of PPARA protein, which is required in fatty acid homeostasis and in the transcriptional regulation of genes that are necessary for maintaining the redox balance during the oxidative catabolism of fatty acids, therefore increasing lipid accumulation during pre-adipocyte differentiation [27]. MIR-519d also suppresses epithelial–mesenchymal transition in gastric cancer via Twist1 and inhibits Wnt/β-catenin signaling pathway [28], while the downregulation of miR-519d by C14orf28 attenuates apoptosis and EMT in colorectal cancer, leading to enhanced carcinogenesis [29]. Interestingly, miR-519d promotes the progression and metastasis of cervical cancer through direct targeting Smad7 [30].
Multiple studies confirmed that miRNAs could regulate the expression of LIMK1 to affect its biological function in different cancer types. For example, miR-20a regulates LIMK1 in anaplastic thyroid cancer and cutaneous squamous cell carcinoma [11, 12]; miR-138 modulates LIMK1 in ovarian and lung cancers [13, 14]. In breast cancer, our recent results showed that miR-200b-3p, miR-429-5p, and miR-143-3p target LIMK1 to impact cellular proliferation, migration, and invasion [15, 16]. These results demonstrate that LIMK1 can be regulated by multiple miRNAs. Whether LIMK1 is regulated primarily by specific miRNA combinations in relation to its differential function is still unknown.
In the present study, we found a new miRNA-miR-519d-3p that regulates LIMK1 in breast cancer cells. To confirm whether LIMK1 is a direct target of miR-519d-3p, vectors containing binding sites of miR-519d-3p and LIMK1 were constructed for study and the corresponding luciferase report assays showed direct binding of miR-519d-3p and 3′-UTR of LIMK1 at the region of 1076–1082 but not at the region of 660–666. Meanwhile, overexpression of miR-519d-3p in MDA-MB-231 and HCC1937 cells decreased the expression of LIMK1 at both mRNA and protein levels. Furthermore, overexpression of miR-519d-3p significantly decreased the expression of CFL1 and phosphorylation of CFL1. These results demonstrate that miR-519d-3p suppresses breast cancer cell growth and motility at least partially via inhibiting the LIMK1/CFL1 signal pathway.
Cofilin family functions as the major substrate of LIMK1. Via phosphorylation of CFL1, LIMK1 suppresses actin-severing activity and therefore impacts actin cytoskeleton organization including on invadopodia actin dynamics. During collective cell migration, LIMK1 is an essential molecule required for migration and invasion by stimulating cancer cells to form an invasive path [22]. The TCGA database also showed that LIMK1 was highly expressed in breast cancer tissues when compared with normal breast tissue. When compared to breast cancer, LIMK1 expression was especially higher in TNBC. This strongly suggests that targeting LIMK1 may be a potential strategy for effectively treating progressive, invasive, and metastatic ENBC and TNBC.
Conclusion
Our results demonstrate that miR-519d-3p suppresses breast cancer cell growth and motility by targeting the LIMK1/CFL1 pathway. MiRNAs, existing in blood, plasma, serum, saliva, urine, and other tissues, are potentially ideal biomarkers for diagnosing cancer, staging lesions and subtypes, monitoring disease progression, and predicting prognosis and therapy response [31]. In future, further characterization of the effect from gain or loss of miR-519d-3p is needed in animal models.
References
Fan L, Strasser-Weippl K, Li JJ, St Louis J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM, Goss PE (2014) Breast cancer in China. Lancet Oncol 15:e279–e289. https://doi.org/10.1016/S1470-2045(13)70567-9
Society AC (2017) Cancer facts and figs. 2017. American Cancer Society, Atlanta
Zhu J, Zhou Q, Tan S (2016) Targeting miRNAs associated with surface expression of death receptors to modulate TRAIL resistance in breast cancer. Cancer Lett 383:154–160. https://doi.org/10.1016/j.canlet.2016.09.021
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Cuberos H, Vallee B, Vourc’h P, Tastet J, Andres CR, Benedetti H (2015) Roles of LIM kinases in central nervous system function and dysfunction. FEBS Lett 589:3795–3806. https://doi.org/10.1016/j.febslet.2015.10.032
Liao Q, Li R, Zhou R, Pan Z, Xu L, Ding Y, Zhao L (2017) LIM kinase 1 interacts with myosin-9 and alpha-actinin-4 and promotes colorectal cancer progression. Br J Cancer 117:563–571. https://doi.org/10.1038/bjc.2017.193
Park GB, Kim D (2017) PI3K catalytic isoform alteration promotes the LIMK1-related metastasis through the PAK1 or ROCK1/2 activation in cigarette smoke-exposed ovarian cancer cells. Anticancer Res 37:1805–1818. https://doi.org/10.21873/anticanres.11515
Li R, Doherty J, Antonipillai J, Chen S, Devlin M, Visser K, Baell J, Street I, Anderson RL, Bernard O (2013) LIM kinase inhibition reduces breast cancer growth and invasiveness but systemic inhibition does not reduce metastasis in mice. Clin Exp Metastasis 30:483–495. https://doi.org/10.1007/s10585-012-9553-6
McConnell BV, Koto K, Gutierrez-Hartmann A (2011) Nuclear and cytoplasmic LIMK1 enhances human breast cancer progression. Mol Cancer 10:75. https://doi.org/10.1186/1476-4598-10-75
Ohashi K, Sampei K, Nakagawa M, Uchiumi N, Amanuma T, Aiba S, Oikawa M, Mizuno K (2014) Damnacanthal, an effective inhibitor of LIM-kinase, inhibits cell migration and invasion. Mol Biol Cell 25:828–840. https://doi.org/10.1091/mbc.E13-09-0540
Xiong Y, Zhang L, Kebebew E (2014) MiR-20a is upregulated in anaplastic thyroid cancer and targets LIMK1. PLoS ONE 9:e96103. https://doi.org/10.1371/journal.pone.0096103
Zhou J, Liu R, Luo C, Zhou X, Xia K, Chen X, Zhou M, Zou Q, Cao P, Cao K (2014) MiR-20a inhibits cutaneous squamous cell carcinoma metastasis and proliferation by directly targeting LIMK1. Cancer Biol Ther 15:1340–1349. https://doi.org/10.4161/cbt.29821
Chen P, Zeng M, Zhao Y, Fang X (2014) Upregulation of Limk1 caused by microRNA-138 loss aggravates the metastasis of ovarian cancer by activation of Limk1/cofilin signaling. Oncol Rep 32:2070–2076. https://doi.org/10.3892/or.2014.3461
Tan Y, Hu H, Tan W, Jin L, Liu J, Zhou H (2016) MicroRNA-138 inhibits migration and invasion of non-small cell lung cancer cells by targeting LIMK1. Mol Med Rep 14:4422–4428. https://doi.org/10.3892/mmr.2016.5769
Li D, Hu J, Song H, Xu H, Wu C, Zhao B, Xie D, Wu T, Zhao J, Fang L (2017) miR-143-3p targeting LIM domain kinase 1 suppresses the progression of triple-negative breast cancer cells. Am J Transl Res 9:2276–2285
Li D, Wang H, Song H, Xu H, Zhao B, Wu C, Hu J, Wu T, Xie D, Zhao J, Shen Q, Fang L (2017) The microRNAs miR-200b-3p and miR-429-5p target the LIMK1/CFL1 pathway to inhibit growth and motility of breast cancer cells. Oncotarget. https://doi.org/10.18632/oncotarget.19205
Zhang J, Kong X, Li J, Luo Q, Li X, Shen L, Chen L, Fang L (2014) miR-96 promotes tumor proliferation and invasion by targeting RECK in breast cancer. Oncol Rep 31:1357–1363. https://doi.org/10.3892/or.2013.2934
Jacob A, Prekeris R (2015) The regulation of MMP targeting to invadopodia during cancer metastasis. Front Cell Dev Biol 3:4. https://doi.org/10.3389/fcell.2015.00004
Goodlad RA (2017) Quantification of epithelial cell proliferation, cell dynamics, and cell kinetics in vivo. Wiley Interdiscip Rev Dev Biol. https://doi.org/10.1002/wdev.274
Deng X, Zhao Y, Wang B (2015) miR-519d-mediated downregulation of STAT3 suppresses breast cancer progression. Oncol Rep 34:2188–2194. https://doi.org/10.3892/or.2015.4160
Xie Q, Wang S, Zhao Y, Zhang Z, Qin C, Yang X (2017) MiR-519d impedes cisplatin-resistance in breast cancer stem cells by down-regulating the expression of MCL-1. Oncotarget 8:22003–22013. https://doi.org/10.18632/oncotarget.15781
Lagoutte E, Villeneuve C, Lafanechere L, Wells CM, Jones GE, Chavrier P, Rosse C (2016) LIMK regulates tumor-cell invasion and matrix degradation through tyrosine phosphorylation of MT1-MMP. Sci Rep 6:24925. https://doi.org/10.1038/srep24925
Mardilovich K, Baugh M, Crighton D, Kowalczyk D, Gabrielsen M, Munro J, Croft DR, Lourenco F, James D, Kalna G, McGarry L, Rath O, Shanks E, Garnett MJ, McDermott U, Brookfield J, Charles M, Hammonds T, Olson MF (2015) LIM kinase inhibitors disrupt mitotic microtubule organization and impair tumor cell proliferation. Oncotarget 6:38469–38486. https://doi.org/10.18632/oncotarget.6288
Kang CG, Im E, Lee HJ, Lee EO (2017) Plumbagin reduces osteopontin-induced invasion through inhibiting the Rho-associated kinase signaling pathway in A549 cells and suppresses osteopontin-induced lung metastasis in BalB/c mice. Bioorg Med Chem Lett 27:1914–1918. https://doi.org/10.1016/j.bmcl.2017.03.047
Kapur R, Shi J, Ghosh J, Munugalavadla V, Sims E, Martin H, Wei L, Mali RS (2016) ROCK1 via LIM kinase regulates growth, maturation and actin based functions in mast cells. Oncotarget 7:16936–16947. https://doi.org/10.18632/oncotarget.7851
Hamam R, Hamam D, Alsaleh KA, Kassem M, Zaher W, Alfayez M, Aldahmash A, Alajez NM (2017) Circulating microRNAs in breast cancer: novel diagnostic and prognostic biomarkers. Cell Death Dis 8:e3045. https://doi.org/10.1038/cddis.2017.440
Martinelli R, Nardelli C, Pilone V, Buonomo T, Liguori R, Castano I, Buono P, Masone S, Persico G, Forestieri P, Pastore L, Sacchetti L (2010) miR-519d overexpression is associated with human obesity. Obesity (Silver Spring) 18:2170–2176. https://doi.org/10.1038/oby.2009.474
Yue H, Tang B, Zhao Y, Niu Y, Yin P, Yang W, Zhang Z, Yu P (2017) MIR-519d suppresses the gastric cancer epithelial-mesenchymal transition via Twist1 and inhibits Wnt/beta-catenin signaling pathway. Am J Transl Res 9:3654–3664
Yang X, Hu Y, Liu Y, Liu W, Zhao X, Liu M, Tang H (2017) C14orf28 downregulated by miR-519d contributes to oncogenicity and regulates apoptosis and EMT in colorectal cancer. Mol Cell Biochem. https://doi.org/10.1007/s11010-017-3049-2
Zhou JY, Zheng SR, Liu J, Shi R, Yu HL, Wei M (2016) MiR-519d facilitates the progression and metastasis of cervical cancer through direct targeting Smad7. Cancer Cell Int 16:21. https://doi.org/10.1186/s12935-016-0298-1
Nassar FJ, Nasr R, Talhouk R (2017) MicroRNAs as biomarkers for early breast cancer diagnosis, prognosis and therapy prediction. Pharmacol Ther 172:34–49. https://doi.org/10.1016/j.pharmthera.2016.11.012
Acknowledgements
This work was supported by the Shanghai Municipal Health Bureau of Shanghai, China through Grant No. 201640097 (to L. Fang), the National Natural Science Foundation of China through Grant No. 82172240 (to L. Fang), Cancer Center Support Grant P30 CA016672 from the United States National Institutes of Health (to The University of Texas MD Anderson Cancer Center), startup funds from MD Anderson Cancer Center (to Q. Shen), and the Duncan Family Institute Seed Funding Research Program (to Q. Shen).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, D., Song, H., Wu, T. et al. MiR-519d-3p suppresses breast cancer cell growth and motility via targeting LIM domain kinase 1. Mol Cell Biochem 444, 169–178 (2018). https://doi.org/10.1007/s11010-017-3241-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3241-4