Abstract
Background
Despite surgical resection and chemoradiation, all patients with GBM invariably recur. Radiological imaging is limited in differentiating tumor recurrence (TR) from treatment-related changes (TRC); therefore, re-resection is often needed. Few studies have assessed the relationship between re-resection histopathology and overall survival (OS). We performed a large retrospective study to analyze the clinical significance of histopathology following re-resection and its influence on genomic sequencing results.
Methods
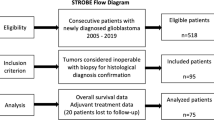
Clinical, radiographic, and histological information was compiled from 675 patients with GBM (2005–2017). 137-patients met the inclusion criteria. IDH1 p.R132H immunohistochemistry was performed in all patients. Next-generation sequencing interrogating 205 tumor-related genes was performed in 68-patients. Molecular alterations from initial and subsequent resections were compared in a subset of cases.
Results
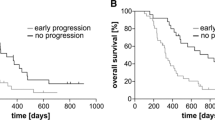
There were no differences in OS (17.3-months TRC vs. 21-months TR, p = 0.881) and survival from progression (9.0 vs. 11.7-months, p = 0.778) between patients with TR and TRC on re-resection. TR patients were more likely to receive salvage radiotherapy (26% vs. 0%) and tumor-treating fields (25% vs. 5%,) after the 2nd surgery than the TRC group (p = < 0.045). There was no correlation between mutations and TRC. IDH status was not predictive of TRC. Fifteen-patients had sequencing results from multiple surgeries without evident differences in genomic alterations.
Conclusions
Histopathologic findings following chemoradiation do not correlate with clinical outcomes. Such findings should be considered during patient management and clinical trial enrollment. Standardization of tissue sampling and interpretation following reoperation is urgently needed. Future work is required to understand the relationship between the mutation profile following TRC and outcomes.


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Omuro A, DeAngelis LM (2013) Glioblastoma and other malignant gliomas: a clinical review. JAMA 310:1842–1850. https://doi.org/10.1001/jama.2013.280319
Delgado-Lopez PD, Corrales-Garcia EM (2016) Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol 18:1062–1071. https://doi.org/10.1007/s12094-016-1497-x
Chen L, Chaichana KL, Kleinberg L, Ye X, Quinones-Hinojosa A, Redmond K (2015) Glioblastoma recurrence patterns near neural stem cell regions. Radiother Oncol 116:294–300. https://doi.org/10.1016/j.radonc.2015.07.032
Ali FS, Arevalo O, Zorofchian S, Patrizz A, Riascos R, Tandon N, Blanco A, Ballester LY, Esquenazi Y (2019) Cerebral radiation necrosis: incidence, pathogenesis, diagnostic challenges, and future opportunities. Curr Oncol Rep. https://doi.org/10.1007/s11912-019-0818-y
Kruser TJ, Mehta MP, Robins HI (2013) Pseudoprogression after glioma therapy: a comprehensive review. Expert Rev Neurother 13:389–403. https://doi.org/10.1586/ern.13.7
Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE (2007) Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol 82:81–83. https://doi.org/10.1007/s11060-006-9241-y
Topkan E, Topuk S, Oymak E, Parlak C, Pehlivan B (2012) Pseudoprogression in patients with glioblastoma multiforme after concurrent radiotherapy and temozolomide. Am J Clin Oncol 35:284–289. https://doi.org/10.1097/COC.0b013e318210f54a
Tihan T, Barletta J, Parney I, Lamborn K, Sneed PK, Chang S (2006) Prognostic value of detecting recurrent glioblastoma multiforme in surgical specimens from patients after radiotherapy: should pathology evaluation alter treatment decisions? Hum Pathol 37:272–282
Woodworth GF, Garzon-Muvdi T, Ye X, Blakeley JO, Weingart JD, Burger PC (2013) Histopathological correlates with survival in reoperated glioblastomas. J Neurooncol 113:485–493. https://doi.org/10.1007/s11060-013-1141-3
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. https://doi.org/10.1016/j.jbi.2008.08.010
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J et al (2019) The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95:103208
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization Classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Zorofchian S, El-Achi H, Yan Y, Esquenazi Y, Ballester LY (2018) Characterization of genomic alterations in primary central nervous system lymphomas. J Neurooncol 140:509–517. https://doi.org/10.1007/s11060-018-2990-6
Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, Schnall-Levin M, White J, Sanford EM, An P et al (2013) Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol 31:1023–1031. https://doi.org/10.1038/nbt.2696
Schwaederle M, Krishnamurthy N, Daniels GA, Piccioni DE, Kesari S, Fanta PT, Schwab RB, Patel SP, Parker BA, Kurzrock R (2018) Telomerase reverse transcriptase promoter alterations across cancer types as detected by next-generation sequencing: a clinical and molecular analysis of 423 patients. Cancer 124:1288–1296. https://doi.org/10.1002/cncr.31175
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 57:289–300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x
Chukwueke UN, Wen PY (2019) Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol. https://doi.org/10.2217/cns-2018-0007
Kim JH, Bae Kim Y, Han JH, Cho KG, Kim SH, Sheen SS, Lee HW, Jeong SY, Kim BY, Lee KB (2012) Pathologic diagnosis of recurrent glioblastoma: morphologic, immunohistochemical, and molecular analysis of 20 paired cases. Am J Surg Pathol 36:620–628. https://doi.org/10.1097/PAS.0b013e318246040c
Haider AS, van den Bent M, Wen PY, Vogelbaum MA, Chang S, Canoll PD, Horbinski CM, Huse JT (2020) Toward a standard pathological and molecular characterization of recurrent glioma in adults: a Response Assessment in Neuro-Oncology effort. Neuro Oncol 22:450–456. https://doi.org/10.1093/neuonc/noz233
Rusthoven KE, Olsen C, Franklin W, Kleinschmidt-DeMasters BK, Kavanagh BD, Gaspar LE, Lillehei K, Waziri A, Damek DM, Chen C (2011) Favorable prognosis in patients with high-grade glioma with radiation necrosis: the University of Colorado reoperation series. Int J Radiat Oncol Biol Phys 81:211–217. https://doi.org/10.1016/j.ijrobp.2010.04.069
Grossman R, Shimony N, Hadelsberg U, Soffer D, Sitt R, Strauss N, Corn BW, Ram Z (2016) Impact of resecting radiation necrosis and pseudoprogression on survival of patients with glioblastoma. World Neurosurg 89:37–41. https://doi.org/10.1016/j.wneu.2016.01.020
Dalle Ore CL, Chandra A, Rick J, Lau D, Shahin M, Nguyen AT, McDermott M, Berger MS, Aghi MK (2019) Presence of histopathological treatment effects at resection of recurrent glioblastoma: incidence and effect on outcome. Neurosurgery 85:793–800. https://doi.org/10.1093/neuros/nyy501
Holdhoff M, Ye X, Piotrowski AF, Strowd RE, Seopaul S, Lu Y, Barker NJ, Sivakumar A, Rodriguez FJ, Grossman SA et al (2019) The consistency of neuropathological diagnoses in patients undergoing surgery for suspected recurrence of glioblastoma. J Neurooncol 141:347–354. https://doi.org/10.1007/s11060-018-03037-3
Jain R (2011) Perfusion CT imaging of brain tumors: an overview. AJNR Am J Neuroradiol 32:1570–1577. https://doi.org/10.3174/ajnr.A2263
Metaweh NAK, Azab AO, El Basmy AAH, Mashhour KN, El Mahdy WM (2018) Contrast-enhanced perfusion MR imaging to differentiate between recurrent/residual brain neoplasms and radiation necrosis. Asian Pac J Cancer Prev 19:941–948
Xu Q, Liu Q, Ge H, Ge X, Wu J, Qu J, Xu K (2017) Tumor recurrence versus treatment effects in glioma: a comparative study of three dimensional pseudo-continuous arterial spin labeling and dynamic susceptibility contrast imaging. Medicine (Baltimore) 96:e9332. https://doi.org/10.1097/MD.0000000000009332
Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A et al (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26:2192–2197. https://doi.org/10.1200/JCO.2007.14.8163
McConnell HL, Schwartz DL, Richardson BE, Woltjer RL, Muldoon LL, Neuwelt EA (2016) Ferumoxytol nanoparticle uptake in brain during acute neuroinflammation is cell-specific. Nanomedicine 12:1535–1542. https://doi.org/10.1016/j.nano.2016.03.009
Henderson F Jr, Brem S, O’Rourke DM, Nasrallah M, Buch VP, Young AJ, Doot RK, Pantel A, Desai A, Bagley SJ et al (2020) (18)F-Fluciclovine PET to distinguish treatment-related effects from disease progression in recurrent glioblastoma: PET fusion with MRI guides neurosurgical sampling. Neurooncol Pract 7:152–157. https://doi.org/10.1093/nop/npz068
Soler DC, Young AB, Cooper KD, Kerstetter-Fogle A, Barnholtz-Sloan JS, Gittleman H, McCormick TS, Sloan AE (2017) The ratio of HLA-DR and VNN2(+) expression on CD14(+) myeloid derived suppressor cells can distinguish glioblastoma from radiation necrosis patients. J Neurooncol 134:189–196. https://doi.org/10.1007/s11060-017-2508-7
Sabari JK, Offin M, Stephens D, Ni A, Lee A, Pavlakis N, Clarke S, Diakos CI, Datta S, Tandon N et al (2019) A Prospective Study Of Circulating Tumor DNA to guide matched targeted therapy in lung cancers. J Natl Cancer Inst 111:575–583. https://doi.org/10.1093/jnci/djy156
Ma J, Benitez JA, Li J, Miki S, Ponte de Albuquerque C, Galatro T, Orellana L, Zanca C, Reed R, Boyer A et al (2019) Inhibition of nuclear PTEN tyrosine phosphorylation enhances glioma radiation sensitivity through attenuated DNA repair. Cancer Cell 35:504.e7-518.e7
Cesare AJ, Reddel RR (2010) Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet 11:319–330. https://doi.org/10.1038/nrg2763
Smogorzewska A, de Lange T (2004) Regulation of telomerase by telomeric proteins. Annu Rev Biochem 73:177–208. https://doi.org/10.1146/annurev.biochem.73.071403.160049
Shay JW, Wright WE (2011) Role of telomeres and telomerase in cancer. Semin Cancer Biol 21:349–353. https://doi.org/10.1016/j.semcancer.2011.10.001
Gao K, Li G, Qu Y, Wang M, Cui B, Ji M, Shi B, Hou P (2016) TERT promoter mutations and long telomere length predict poor survival and radiotherapy resistance in gliomas. Oncotarget 7:8712–8725. https://doi.org/10.18632/oncotarget.6007
Khurana R, Rath S, Singh HB, Rastogi M, Nanda SS, Chauhan A, Kaif M, Hussain N (2020) Correlation of molecular markers in high grade gliomas with response to chemo-radiation. Asian Pac J Cancer Prev 21:755–760
Yao Q, Cai G, Yu Q, Shen J, Gu Z, Chen J, Shi W, Shi J (2018) IDH1 mutation diminishes aggressive phenotype in glioma stem cells. Int J Oncol 52:270–278. https://doi.org/10.3892/ijo.2017.4186
Christians A, Adel-Horowski A, Banan R, Lehmann U, Bartels S, Behling F, Barrantes-Freer A, Stadelmann C, Rohde V, Stockhammer F et al (2019) The prognostic role of IDH mutations in homogeneously treated patients with anaplastic astrocytomas and glioblastomas. Acta Neuropathol Commun. https://doi.org/10.1186/s40478-019-0817-0
Rao AM, Quddusi A, Shamim MS (2018) The significance of MGMT methylation in Glioblastoma Multiforme prognosis. J Pak Med Assoc 68:1137–1139
Binabaj MM, Bahrami A, ShahidSales S, Joodi M, Joudi Mashhad M, Hassanian SM, Anvari K, Avan A (2018) The prognostic value of MGMT promoter methylation in glioblastoma: a meta-analysis of clinical trials. J Cell Physiol 233:378–386. https://doi.org/10.1002/jcp.25896
Barthel FP, Johnson KC, Varn FS, Moskalik AD, Tanner G, Kocakavuk E, Anderson KJ, Abiola O, Aldape K, Alfaro KD et al (2019) Longitudinal molecular trajectories of diffuse glioma in adults. Nature 576:112–120. https://doi.org/10.1038/s41586-019-1775-1
Alter BP (2002) Radiosensitivity in Fanconi’s anemia patients. Radiother Oncol 62:345–347
Taylor AM, Harnden DG, Arlett CF, Harcourt SA, Lehmann AR, Stevens S, Bridges BA (1975) Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature 258:427–429. https://doi.org/10.1038/258427a0
Wang TM, Shen GP, Chen MY, Zhang JB, Sun Y, He J, Xue WQ, Li XZ, Huang SY, Zheng XH et al (2019) Genome-wide association study of susceptibility loci for radiation-induced brain injury. J Natl Cancer Inst 111:620–628. https://doi.org/10.1093/jnci/djy150
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number K08CA241651 (LYB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Study design: AP, LYB, and YE. Data Recollection: AP and AD. Data analysis: AD and PZ. Manuscript writing: AP, AD, and YE. Manuscript revision and editing: AP, AD, NT, LYB, and YE. Study Supervision: LYB and YE. Approved final manuscript: all authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This retrospective study was approved by the institutional review board of The University of Texas Health Science Center at Houston and Memorial Hermann Hospital, Houston, TX following the 1964 Helsinki Declaration and its later amendments.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Patrizz, A., Dono, A., Zhu, P. et al. Tumor recurrence or treatment-related changes following chemoradiation in patients with glioblastoma: does pathology predict outcomes?. J Neurooncol 152, 163–172 (2021). https://doi.org/10.1007/s11060-020-03690-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-020-03690-7